Еffective patenting of agents for the specific prophylaxis of infectious diseases
EFFECTIVE PATENTING OF AGENTS FOR THE SPECIFIC PROPHYLAXIS OF INFECTIOUS DISEASES
Vyatka State Agricultural Academy, Kirov, 610017
The practice of patenting agents for the specific prophylaxis of infectious diseases, carried out in the 1980s—1990s by leading biotechnology organizations, was investigated. It was shown that the nature and strategy of the patent policy of a biotechnological organization in developing the indicated agents might be determined on, the basis of technologies developed by it and rival organizations, patterns in the patenting of individual objects, and the biotechnological peculiarities of the pathogens.
At the present time the leading biotechnological organizations (Genentech, Inc., Inst. Pasteur, Monsanto, etc.) hold patents in virtually all the industrially developed countries. As a result of the patents, a new type of colonial dependence of countries with less of a technological potential on the countries that possess high technologies is forming [1]. It is also extremely important that the patents are capable of maintaining prices for drags at a level significantly exceeding their actual cost [2]. Russian researchers have been operating under conditions of patent law comparatively recently, since 1991, and not all of them have taken proper cognisance of the fact that under the modem conditions there is no sense in developing a new high-tech product without clever patent protection of it. At the same time, the leading foreign developers of biotechnological products are making no effort to reveal the secrets of their activity on the market of patents and licenses. The patenting of inventions "randomly" will be simply ineffective in the best case, and in the worse case will lead to a senseless expenditure of resources, legal disputes, etc.
This work is aimed at aiding developers in our country to select their own strategy in the patenting of vaccines, and also new agents of specific prophylaxis that are replacing them, excluding the immune response from the mechanism of protection from pathogens of infectious diseases.
METHOD OF INVESTIGATIONS
The practice of patenting agents for the specific prophylaxis of infectious diseases, carried out in the 1980—1990s by leading biotechnological organizations, and published works of domestic and foreign specialists on the legal and commercial aspects of the patenting of biotechnological inventions [1—11] were analyzed. An investigation was made of inventions possessing broad patent protection (vaccine, antigen, adjuvant, etc.) and also of inventions that protect key technical decisions on agents of specific prophylaxis of AIDS, influenza, hepatitis B, hemophilia, pertussis, and certain other infections, which we have regarded as leading objects [10]. To study the subjects of development of individual technologies we used the methodology of logical system analysis [12]. The technical solutions created during the design of such agents were considered as an aggregate of elements in a definite interrelationship and constituting a certain unity (integrity), i.e., a system. A given system was dissected into individual subsystems, representing competing, alternative approaches (standard methods), and the potentialities of an arbitrary biotechnological firm for the creation of technical decisions were evaluated. Then the principles of functioning and the structure of effective patentable technical solutions, as well as effective approaches to their patent protection, were predicted. In the text, in a reference to a patent the abbreviated name of the patent authority and the number of the patent document are indicated. References to individual patents, an acquaintance with which might be very useful, are given in the list of sources used.
POTENTIALITIES OF AN ARBITRARY BIOTECHNOLOGICAL ORGANIZATION FOR THE DEVELOPMENT OF PATENTABLE TECHNICAL SOLUTIONS
Figure 1 shows the limits of development of technologies for the specific prophylaxis of infectious diseases. The development of individual key technologies (indicated by a double line) provides an organization with a definite range of potentialities for the patenting of its own inventions [10].
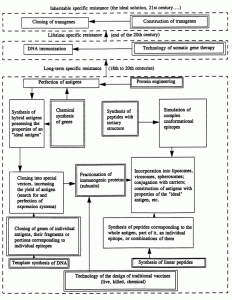 Fig. 1. Limits of development of technologies for the specific prophylaxis of infectious diseases.
Fig. 1. Limits of development of technologies for the specific prophylaxis of infectious diseases.
The technologies of the design of traditional vaccines presupposed the construction of live, killed, and chemical vaccines [13]. The methodological level used for the design of the first two types of vaccines is more characteristic of the end of the 19th century and the beginning of the 1950s. The objects of protection are vaccine strains, virulent strains of a pathogen capable of attenuation, and for killed vaccines — compositions of them with adjuvants, preservatives, and lymphokines; individual technological processes supporting the production of microbial biomass; nutrient media and components for them; means of quality control of vaccines, nutrient media, etc. [9]. The methodological level used for the creation of chemical vaccines is characteristic of the period from the beginning to the middle of the 1970s. Antigens, as a rule, are produced from the culture medium (toxins, slime, enzymes) or from killed cells. In individual cases crude complexes of lipids, proteins, and polysaccharides, uncharacterized with respect to chemical and physicochemical structure and called "protective antigens," "O-antigens," "complexes of membrane proteins," etc. [8] become the objects of protection. At the present time vaccines for veterinary purposes are patented within the framework of these technologies.
Fractionation of immunogenic proteins (subunits) of microorganisms. The development of technology in the form of various methods of chromatographic separation of proteins presupposes an empirical search for protective antigens (their epitopes) among proteins and glycoproteins of viruses and bacteria. As a rule, the antigens included in vaccines are characterized in the patent applications according to physicochemical and immunological properties permitting their identification in mixtures of other biopolymers. Patentable inventions solve problems of reducing the toxicity of antigenic proteins and increasing their immunogenicity. At the end of the 1980s and the beginning of the 1990s, the possibility of producing species-specific immunity (antigens based on fusion proteins of viruses, modified lipid A of bacteria, and epitopes of pore-forming proteins; US 4861707, US 5141867, EP 0244748, WO 94/16082), as well as the creation of effective protection from aerogenic infection (vaccines based on live vectors and porins), became the main criterion in the selection of an antigen for patenting [14, 15]. The objects of patenting are compositions or compounds including an antigen and components that promote its effective presentation to the immune system [9].
Cloning of individual genes of antigens and their fragments. The technology from the end of the 1970s is used for the construction of vaccines against pathogens of infectious diseases representing DNA viruses, bacteria, and rickettsias. Within the framework of the technology, successive recloning of DNA fragments is performed, aimed at the production of the portions that determine the synthesis of conservative and highly immunogenic antigens (epitopes), and perfection of approaches to the presentation of the antigen to cells of the immune system (FR 2587720,2600079,267518, WO 88/00311, 92/22641). For this purpose the genes of antigens are cloned into viral and bacterial vectors, HBsAg particles [14]. Another approach, implementing the inventive potential vested in this technology, is an increase in the level of expression and secretion of the genes of cloned antigens. It finds expression in the appearance of patents for expression vectors and methods of purifying antigens, based on fine methods of fractionation of biopolymers (EP 0343132). The search for (and, correspondingly, patenting of) new producers (tissue cultures of eukaryotes, methylotrophic yeasts, transgenic plants and animals, etc.; JP 74990, EP 255785, EP 0340837) are carried out within the framework of the same technology. Antigens synthesized in different expression systems are also included among compositions that enhance the immune response [9].
Template synthesis of DNA. The development of the technology permits an organization to clone the genes of RNA viruses [8]. The potentialities and limitations are the same as we noted in the discussion of the preceding technology. Both characterize the methodological level of the 1980s.
Chemical synthesis of genes began to be used most actively for the production of genes of antigens in the second half of the 1980s. The development of the technology makes it possible to construct genes of antigens that consist of several epitopes [9]. The construction of antigens possessing the properties of the "ideal antigen" for a given microorganism — host system is most realistic within this framework. But at the same time, the reaching of this level by an organization is a unique indicator of exhaustion of the potentialities of the traditional recombinant DNA technology and, correspondingly, an imminent decrease in the patent activity on the object.
Protein engineering. The technology has been used since the second half of the 1980s to remove protease cleavage sites from antigenic peptides, to modify the conformation of epitopes, and to reduce the toxicity of antigens (EP 0155146). Antigens are the objects of patenting [9]. However, just as in the use of chemical synthesis of genes, the use of protein engineering by developers for the creation of new technical solutions is an indication of an imminent decrease in patent activity on the object.
Synthesis of linear peptides. Vaccines based on synthetic linear peptides have been patented since the beginning of the 1980s. The development of the technology of peptide synthesis permits an organization to patent synthetic analogs of protective epitopes, including those simultaneously corresponding to several natural antigens. The low immunogenicity of synthetic peptides prompts developers to improve the methods of their presentation to the immune system, for example, by enclosing them in liposomes, forming conjugates and compositions with immunomodulators, and introducing portions recognized by T and/or A lymphocytes, etc. into their structure [13, 14].
Synthesis of peptides with a tertiary structure. This technology denotes the construction of peptides with an artificially imparted conformation (cyclization of peptides, joining of two or more peptides by cross-links, synthesis of branched peptides, etc.; US 4639371, US 4778784). The technology has been used since the first half of the 1980s for the reproduction or simulation of complex epitopes [16]. It potentialities have not been investigated completely enough; however, an analysis of the dynamics of patenting shows that the appearance of patents protecting antigenic polypeptides of this type is also a signal of a possible abandonment of the production of synthetic vaccines against a given pathogen in the near future and the transition to other technologies for the construction of means of specific prophylaxis.
DNA immunization. The method is based on the introduction of plasmid DNA capable of expressing the genes of antigens into a macroorganism. As one of the technologies for somatic gene therapy, DNA immunization has been actively developed since the beginning of the 1990s (WO 94/2197). A common factor with the traditional immunization with live vaccines is the fact that the development of the immune response occurs in response to endogenously synthesized antigenic proteins; however, they are expressed in the tissues (skin, muscles, spleen, etc.) of the immunized object without the development of the infection process. Theoretically, a lifetime resistance to several pathogens of infectious diseases can be achieved within the framework of this technology, by a single injection of plasmid DNA [17]. Reaching this level is characteristic of organizations that use the recombinant DNA technology and work with vectors of eukaryotic cells.
Now let us examine the technologies that exclude the immune response from the mechanism of protection from the pathogen of an infectious disease.
Blocking of binding of the pathogen to receptors. A specific protective effect is achieved by shielding a receptor on the surface of the sensitive cell with a lectin-like compound specific for the pathogen (toxin) [18]. The design of the preparations takes the approach of empirical selection (synthesis) of relatively nontoxic compounds (plant glycosides, B-subunits of toxins) possessing a receptor binding constant greater than that of the pathogen (toxin) or equal to it [18, 19].
Binding of the pathogen to intracellular fluids of the organism. The technology proposes the introduction of compounds capable of blocking the action of the agent by binding to its conservative structures, involved in specific recognition [20]. To counteract pathogens whose persistence is accompanied by a phenomenon of antibody-dependent enhancement of the infection, specific blocking compounds are used as analogs of antibodies. For the same reason, they are incorporated into the membranes of liposomes, and into the liposomes themselves — a chemopreparation (WO 90/04414, EP 0385909, WO 91/04050). Another approach to patenting is the creation of derivatives of such compounds with an increased biological half-life (WO 90/08198, WO 91/00360). At the same time, nucleotide sequences that code for fragments of protein receptors, recombinant plasmids and vector DNA molecules including these sequences, producer strains capable of producing plasmids, and strains capable of purifying receptor proteins and their derivatives are patented.
Use of the interference phenomenon. The phenomenon was empirically detected by L. Pasteur, and he used it to block the infection process caused by the rabies pathogen [21]. A specific prophylactic and therapeutic effect is achieved by the introduction of a weakened virus strains that competes with the virulent strain for receptors of the target cells. Blocking of infection is achieved even before the formation of antibodies. Interest in the patenting of developments created within the framework of this technology began to increase with the end of the 1980s on account of failures that accompanied the design of vaccines against a number of new diseases.
Oligonucleotide and antisense inhibition of genes of the pathogen. Both approaches have been developed since the end of the 1980s [22, 23]. Originally oligonucleotides were patented as chemotherapeutic agents. It was suggested that, when introduced into the organism, these compounds penetrate into cells and bind to definite structures of proteins and nucleic acids (mRNA for antisense oligonucleotides) of the pathogens. The development of the technology as applied to a concrete pathogen took the path of increasing the resistance of nucleotides to nucleases and also expanding the assortment of blockable structures and selecting those of them, binding to which most effectively blocks the development of the infection process. Antisense oligonucleotides exhibited greater effectiveness than oligomeric nucleotides of the same length [23]. Therefore, later patents protect more effective oligonucleotides with a characterized tertiary structure (WO 94/08004). However, as the complexity of such structures increased, their further development was held back. The use of antisense sequences reached the level of somatic gene therapy. This was preceded by patents for conjugates of antisense sequences with ligand molecules that recognize receptors on the surface of the target cells. The effect of receptor endocytosis has been actively used inpatentable constructs capable of delivering DNA sequences to the cytoplasm of definite cells and transcribing the antisense RNA there (US 5324643). At the present time both constructs targeted against individual pathogens (particular technological solutions of already known problems) and those capable of intracellular regulation of the expression of the genes of antisense nucleic acids are being actively patented.
Catalytic cleavage of mRNA of individual proteins. On the whole, the patenting of ribozymes [24] is observing the same patterns as noted for antisense constructs.
Specific regression of genes of the pathogen is achieved by the intracellular expression of the genes of regulatory proteins (WO 93/23569, WO 94/01549). The approach has been developed since the end of the 1980s within the framework of the technology of somatic gene therapy. The induction of endogenous genes of regulatory proteins, as a rule, is accomplished with products of the genes expressed by the pathogen (WO 93/05147).
Destruction of infected cells by a specific cytotoxin. Various conjugates of ligands (antibodies, CD4 protein, B-subunits of individual toxins, and biologically active substances) and cytotoxins that selectively destroy infected cells [25] were patented actively at the end of the 1980s (WO 90/04414, EP 0385909, WO 91/04050). However, later the interest in the legal protection of such compounds decreased.
Destruction of infected cells by expression of an exogenous cytolytic gene. In the technical solutions announced, there is a combination of the two approaches described above. The induction of genes of cytolytic proteins (ricin A or herpesvirus type 1 thymidine kinase), introduced into the cytoplasm of cells sensitive to the pathogen of the infectious disease, is accomplished with products of genes expressed by the pathogen itself [26].
Intracellular lysis of the pathogen consists of cytoplasmic lysis of the pathogen "by parts." In the technical solutions patented since the beginning of the 1990s, "assembly" of the virion is blocked by intracellularly synthesized structures capable of ligand-receptor (specific) interaction with proteins of the virus. These same structures direct individual viral proteins into lysosomes (WO 94/16672). In subsequent technical solutions, in addition to blocking assembly of the virion, problems of destruction (blocking) of mRNA synthesized on the DNA of the provirus are solved [27].
Construction and cloning of transgenes. Within the framework of both technologies, the achievement of an "ideal technical result," i.e., the creation of an individual possessing inheritable resistance to the pathogen of an infectious disease, is realistic (EP 0462215). The technologies may become the only effective ones in the case of catastrophic infection of the population with retrovirus and other pathogens.
The patterns in the appearance of patents are shown in Fig. 2. Patenting of an object stops hi the following cases.
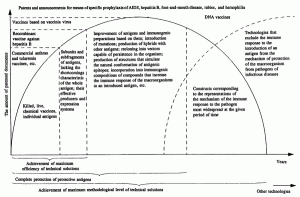 Fig. 2. Patterns in the patenting of vaccine preparations.
Fig. 2. Patterns in the patenting of vaccine preparations.
Achievement of complete patent protection of an object. As applied to vaccines, this pattern is a reflection of an empirical approach to the selection of antigens. Chronologically, whole cells of the pathogen or some most immunogenic strain of it are patented first, then antigenic proteins, glycoproteins, and other polymer structures of the microorganism of various localization, capable of inducing protective immunity in the macroorganism. For example, for a number of viruses, vaccines based on live and killed cells were patented, then individual surface proteins, and finally internal (matrix) proteins more difficult to purify and less immunogenic, as well as their combinations with surface proteins. Patenting stopped after all the effective antigenic structures of the pathogen and their combinations had been characterized and announced.
Achievement of the maximum methodological level of patentable technical solutions. The pattern is manifested in the successive enlistment of methodologically more complex approaches (chemical synthesis of genes, protein engineering, synthesis of peptides with a tertiary structure, DNA immunization, oligonucleotide and antisense inhibition of genes of the pathogen, etc.) into the construction of means of specific prophylaxis, which is the result of an incorrectly selected principle for solving the technical problem. After exhaustion of the potentialities of these technologies, patenting on the object stops. The result of the efforts of a large number of developers most often becomes the "ideal technical solution." For vaccines this is the antigenic structure corresponding to the ideas of the mechanism of the immune response to the pathogen most widespread at the given period of time, but not necessarily providing effective protection from it.
Achievement of maximum effectiveness. Patenting on an object ceases or becomes minimal after the creation of a technical solution satisfying the needs of society at the given moment of time. Such solutions include live plague, anthrax, tularemia, and smallpox vaccines and a number of anatoxins developed in the 1920s to 1940s [13]. Their further improvement, solving individual particular problems, for example, a decrease in reactogenicity, has worsened other consumer properties, such as immunogenicity, low cost, and prolonged protective effect.
The first two patterns can be traced using an evolutionary model (Fig. 3), reflecting the activity of the Institut Pasteur (France) on the development (patenting) of means of specific prophylaxis of HIV infection. At the same time it makes it possible to explain the causes of the loss of leadership by the Institut Pasteur in the developments of vaccines against HIV.
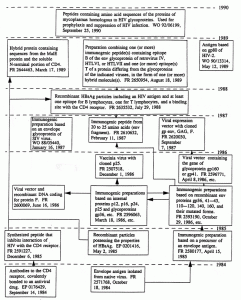 Fig. 3. Evolutionary model of the construction of vaccines against HIV at the Institut Pasteur.
Fig. 3. Evolutionary model of the construction of vaccines against HIV at the Institut Pasteur.
According to this model, the construction of vaccines began with the isolation of an envelope antigen and the production of a chemical vaccine based on it (FR 2571768, FR 2580177). Since 1986 the Institut Pasteur has patented only genetically engineered vaccines based on envelope and internal proteins, as well as a group-specific antigen. Most of the patent documents that have come out, announced in 1986 and later, protected insignificant improvements aimed at reducing antigenic drift and decreasing side effects of immunization. The development of HIV vaccines was accompanied by the appearance of patents with a successively decreasing volume of rights claimed (see, for example, the pioneering FR 2571768 and FR 2580177, but then come small improvements: Fr 2610632, FR 2593910, FR 2620030, EP 0201416, etc.). Comparing the evolutionary model with a scheme of the HIV genome, it becomes evident that the technical solutions patented by the Institut Pasteur during the period from 1984 to 1990 covered virtually all the genes that code for proteins (combinations of them, individual fragments and point mutations) possessing the ability to induce an immune response in the macroorganism. Virtually all of the vectors known in the first half of the 1980s were used for cloning the genes. Analogous investigations were conducted by the Institut Pasteur at the same time with HIV-2 and SIV. The unique result of the work was the creation in 1988 of a vaccine preparation based on the recombinant protein HBsAg, which combines several particular technical solutions (FR 2635532). The preparation was an antigen inducing the production of virus-neutralizing antibodies, regions for antigen recognition by T and A lymphocytes, and sites of binding to the CD4 receptor, i.e., the "ideal antigen." It was suggested that its use could not only block penetration of the virus through the CD4 receptor (according to two mechanisms!) but also neutralize the virus before its interaction with sensitive cells. However, a year later the construction of HIV vaccines on the basis of the antigens announced previously stopped (WO 92/06199).
Thus, the construction of vaccines against HIV at the Institut Pasteur, passing through the stages shown in Fig. 2, ended with the development of the "ideal antigen." The data cited show an exhaustion of the potentialities of the technologies used at the institute for the construction of vaccines on the basis of structural antigens of HIV. At the same time, complete patent protection of the object was achieved; however, no effective technical solution was created.
PREDICTION OF THE PRINCIPLE OF FUNCTIONING AND STRUCTURE OF EFFECTIVE TECHNICAL SOLUTIONS
Selection of the principle of functioning of the object. The selected principle of functioning should permit the creation of progressive, profitable technical solutions that will enjoy a long demand in the market for means of specific prophylaxis.
Table 1, constructed according to the type of P. Roller's matrix [28], systematizes the principles of functioning of protective technical solutions used for the creation of means of specific prophylaxis and the treatment of dangerous infectious diseases. Types of incoming influences on the macroorganism — types of persistence of the pathogen critical for the development of such agents — are indicated in the vertical columns of the matrix. The resultant actions, providing the developer with effective functioning of the technical solution in the case of a given initial influence, are indicated in the horizontal columns.
Table 1
Principles of Functioning of Protective Technical Solutions Used for the Creation of Means of Specific Prophylaxis and Treatment of Dangerous Infectious Diseases
|
Initial influence on macroorganisms
|
Resultant action that must be ensured for effective functioning of the technical solution |
|||
|
Neutralization of the pathogen in extracellular fluids of the organism (1) |
Inhibition of penetration of the pathogen into sensitive cells (2) |
Inhibition of intracellular reproduction of the pathogen (3) |
||
|
Persistence in the macroorganism (A) |
Binding of the pathogen to antibodies. Binding of the pathogen to water-soluble receptors. Binding of the pathogen by local immunity factors |
Blocking of recognition receptors. Use of the interference phenomenon |
Destruction of infected cells with the aid of cellular immunity factors |
|
|
Persistence accompanied by the phenomenon of antibody-dependent enhancement of infection (B) |
Binding of the pathogen to water-soluble receptors |
Blocking of recognition receptors. Use of the interference phenomenon |
Destruction of infected cells by expression of an exogenous cytolytic gene. Oligonucleotide and antisense inhibition of genes of the pathogen. Specific repression of genes of the pathogen. Destruction of infected cells by a specific cytotoxin. Catalytic cleavage of mRNA of individual genes. Intracellular lysis of the pathogen |
|
|
Persistence with retention of provirus in the genome of the macroorganism (C) |
|
|
Cloning of strains of animals that do not contain provirus (decrease in symptoms of the disease; see B, column 3) |
|
In the creation of means of specific prophylaxis and therapy of infections caused by microorganisms, the main routes of whose spread in the macroorganism are hematogenic and lymphogenic (and also in the case of damage by biological toxins), the use of the principles of functioning of technical solutions shown in line A of the matrix (see Table 1, for biological toxins — columns 1 and 2) will be effective. If the developers undertake to neutralize the pathogen (toxin) before its interaction with the sensitive cell, then the principle is selected at the intersection of line A and column 1. But this selection is significantly smaller if the persistence of the pathogen is accompanied by the phenomenon of antibody-dependent enhancement of infection (HIV, Dengue fever pathogens, etc.) — the intersection of line A and column 1. The presence of patents on so-called HIV vaccines should not lead the researcher to error. An analysis of the descriptions of the inventions reveals that the optimism of the developers of such vaccines was stimulated by the production of high titers of antibodies against the proteins of the virus in mice and rabbits [29]. However, investigations of infected people in all cases made it possible to follow only the history of the resistance of the immune system, but never its victory [30].
For pathogens that persist in the presence of provirus (retrovirus) in the genome of the macroorganism, technical solutions capable of neutralizing the pathogen in the blood of the macroorganism or inhibiting its penetration into sensitive cells are of virtually no value, since they are spread mainly as a result of intercellular contact.
The available data, unfortunately, indicate the impossibility of complete elimination of provirus from the genome of the macroorganism [31]. Therefore, at the intersection of line A with column 3 we indicate a technology hypothetical for humans but already realistic for animals — the cloning of virus-free (prion-free) strains of transgenic animals.
Let us take up in more detail the selection of variants of technical solutions against pathogens, with respect to which no effective vaccines have been created.
Prediction of the structure of effective technical solutions. Let us represent in the form of a matrix the variants of technical solutions used for the construction of means of specific prophylaxis of infectious diseases (Table 2). Let us compare the known technical solutions (vertical columns); let us reveal the boxes of the matrix that are most often filled and those of them that remain empty (horizontal columns).
Table 2
Morphological Matrix of Variants of Technical Solutions Used in the 1980s and 1990s in the Construction of Means of Specific Prophylaxis of Infectious Diseases
|
Pathogen
|
Traditional vaccines
|
DNA vaccines |
Technology Of somatic Gene therapy. excluding an immune response of the macro-organism to introduction of an antigen |
||||||||||||
|
Types of antigens
|
Increase in immunogenicity of the antigen by means of |
||||||||||||||
|
Functionally characterized pepti-des and their sub-units |
Live strains, including recombinant |
Killed strains |
Cloning in viral vectors |
Conjugation with a carrier |
Formation of a noncovalent complex with a carrier |
Incorporation into surface structures of bacteria |
Addition of lymphokines to the composition |
Simulation of the conformation of natural antigens |
Incorporation into a multiphase system of the "liposome" type |
Incorporation into polymer compositions |
Incorporation of structures that selectively interact with immunocompetent cells |
Incorporation into a Multiphase system of the "oil—water", "water 1—oil" type |
|||
|
AIDS* |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
– |
+ |
+ |
|
Influenza |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
– |
+ |
– |
|
Hepatitis A |
+ |
– |
– |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
– |
+ |
+ |
|
Foot-and-mouth disease |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
– |
– |
– |
+ |
+ |
– |
|
Pertussis |
+ |
+ |
+ |
– |
+ |
+ |
+ |
– |
– |
+ |
– |
– |
– |
+ |
– |
|
Rabies |
+ |
+ |
+ |
+ |
+ |
+ |
– |
+ |
+ |
– |
– |
+ |
– |
+ |
– |
|
Plague |
+ |
+ |
+ |
– |
+ |
+ |
+ |
+ |
– |
+ |
– |
– |
– |
– |
– |
|
Anthrax |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
– |
+ |
– |
– |
– |
– |
– |
|
Hemophilia |
+ |
+ |
+ |
– |
+ |
+ |
+ |
– |
+ |
– |
– |
– |
– |
+ |
– |
|
Bacillus pyocyaneus infection* |
+ |
+ |
+ |
– |
+ |
– |
+ |
– |
– |
– |
– |
– |
– |
– |
– |
|
Tetanus |
+ |
– |
– |
+ |
+ |
+ |
+ |
– |
– |
– |
+ |
– |
– |
– |
– |
|
Melioidosis* |
+ |
+ |
+ |
– |
+ |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
|
Glanders* |
+ |
+ |
+ |
– |
– |
– |
– |
+ |
– |
– |
– |
– |
– |
– |
– |
|
Dengue fever* |
+ |
+ |
+ |
+ |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
|
Venezuelan equine encephalitis |
– |
+ |
+ |
+ |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
|
Legionnaires' disease* |
+ |
+ |
+ |
– |
– |
– |
– |
|
– |
– |
– |
– |
– |
– |
– |
|
Q-Fever |
+ |
– |
– |
+ |
– |
– |
– |
– |
– |
+ |
– |
– |
– |
|
– |
|
Ebola fever* |
– |
– |
+ |
+ |
– |
– |
– |
– |
– |
– |
+ |
– |
– |
– |
– |
|
Yellow fever |
+ |
+ |
– |
+ |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
|
Lassa fever* |
+ |
– |
+ |
+ |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
|
Hantaan fever* |
+ |
+ |
– |
– |
+ |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
|
Tularemia |
+ |
+ |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
|
Marburg fever* |
– |
– |
+ |
+ |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
|
Rift Valley fever* |
+ |
+ |
– |
+ |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
Note: *Effective agents for specific prophylaxis have not been created.
From the morphological matrix it follows that developers of vaccines place the greatest hopes in functionally characterized peptides, their subunits (surface proteins of viruses and bacteria, toxins), synthetic and genetically engineered peptides corresponding to immunogenic epitopes, and also live attenuated strains of the pathogen, including those obtained as a result of genetic engineering manipulations. The following approaches have become the most widespread for increasing the immunogenicity of antigens to be incorporated into vaccines (with the exception of adsorption on a mineral carrier, which had already become traditional): conjugation with a carrier, cloning in bacterial and viral vectors, and the formation of noncovalent complexes of the antigen and carrier [9]. At the same time, such relatively old approaches to increasing the immunogenicity of antigens as incorporation into multiphase systems and the formation of "antigen—antibody" complexes (US 4847080, EP 0243913) have not found wide use. An analysis of the dynamics of patenting gives evidence of a cessation of developments of new technical solutions within the framework of these technologies at the end of the 1990s. This is an indirect indication of their ineffectiveness. An analysis of the structure of the patent documents shows that such approaches as "addition of lymphokines to the composition" and "incorporation of structures that interact selectively with immunocompetent cells" lost their independent significance at the beginning of the 1990s and were converted to particular methods of construction of genetically engineered vaccines, in which the genes of lymphokines are encoded either in a weakened strain (WO 88/00971) used for immunization or, together with the gene of the antigen, into a vaccine-forming vector (for example, into vaccinia virus; WO 92/12240). The antigens themselves are constructed in such a way as to have recognition sites for T and A lymphocytes (the "ideal antigen" [32]). The same also occurred with polymer compositions. At the end of the 1980s they were no longer considered only as an "antigenic depot," but the degradation products of polymers were used as immunostimulators (WO 87/06129, WO 88/02262). Lymphokines are incorporated into them for the same purpose (WO 88/02262). But at the same time, the complexity of the compositions increases, the number of compounds included in the compositions increases, and the number of classes of compounds of different kinds increases, which makes their patent protection unstable. An analysis of the interrelationships of the technical solutions used in the construction of vaccine preparations (Fig. 4) provides evidence that the interest in various immunomodulating compositions (as well as in other "non-live" vaccines) will not be long-lasting. A fundamental limitation is the inability of such vaccines to effectively induce CD8+ cytotoxic lymphocytes, i.e., the cellular link of immunity [13, 17].
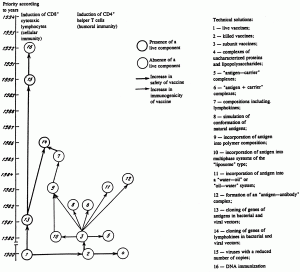 Fig. 4. Interrelationship of patentable technical solutions in the construction of vaccine preparations.
Fig. 4. Interrelationship of patentable technical solutions in the construction of vaccine preparations.
Now let us compare the "horizontal columns" of the matrix (see Table 2). Noteworthy is the exhaustion of alternatives for the development of vaccines against AIDS, hepatitis B, foot-and-mouth disease, pertussis, rabies, plague, anthrax, and hemophilia. At the same time, a transition to individual technologies of somatic gene therapy (DNA immunization and prophylaxis of AIDS, hepatitis B, influenza, foot-and-mouth disease, rabies, hemophilia, pertussis) is observed. At the same time, relatively few modern technical solutions have been used in the construction of vaccines against certain other pathogens of infectious diseases (glanders, melioidosis, hemorrhagic fevers).
To rule out multivariance in the creation of effective technical solutions, we shall proceed from the following minimum requirements:
1. the antigen should induce species-specific immunity—this permits the functioning of the technical solution in a natural focus of the infectious disease in the presence of antigenic drift of the pathogen or when rare serotypes of it appear [13];
2. the cellular link should predominate in the immune response, since precisely it is the most effective in the prophylaxis of disease caused by Gram-negative microorganisms and viruses. Moreover, a peculiarity of cellular immunity is the fact that the response occurs on many epitopes of the same pathogen. This makes it possible to be protected from pathogens that are characterized by antigenic variability [13, 17];
3. immunization should prevent aerogenic infection as the most dangerous of the possible mechanisms of spread of pathogens of infectious diseases among humans and domestic animals, and when they are used as biological warfare agents [13, 33].
An analysis of the scientific literature provides evidence that species-specific immunity can be induced by pore-forming proteins, derivatives of lipid A of Gram-negative bacteria, and also fusion proteins and internal (matrix) proteins of viruses [14, 15]. Induction of cellular immunity of a macroorganism occurs most effectively in response to the introduction of the same ?iaa-forming proteins, fusion proteins of viruses, live vaccines, vaccines based on viral and bacterial vectors, and DNA vaccines [14, 17]. Protection from aerogenic infection has been shown on experimental animals immunized with porins, with live vaccines based on bacterial and viral vectors, and also with DNA vaccines [14, 15], i.e., paradoxical as this might seem, the selection of antigens for vaccines is limited to individual surface proteins of microorganism and matrix proteins of viruses, and the vaccines themselves should represent either live organisms or simulators of them (DNA vaccines). The internal laws of construction of vaccines against Gram-negative microorganisms are shown in Fig. 5. A bank of undesirable effects that arise during the construction of vaccine preparations is given in Table 3.
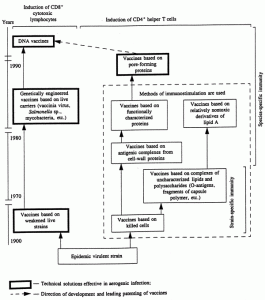 Fig. 5. Internal laws of construction of vaccines against Gram-negative microorganisms.
Fig. 5. Internal laws of construction of vaccines against Gram-negative microorganisms.
Table 3
Bank of Undesirable Effects that Arise During the Construction of Vaccine Preparations
|
Solution |
Undesirable effect |
Elimination of undesirable effect (the ordinal number of the solution (see left columns) is indicated in parenthesis) |
|
1. "Live" vaccines |
Reversion to pathogenic forms. Limited lifetime of the live component. Contamination by extraneous pathogens. Possibility of development of an infection process in young persons and immune deficient patients |
Inactivation of the live component (2). Use of bacterial exposure vectors (13). DNA immunization (16) |
|
2. Inactivated vaccines (killed cells, individual antigens or their subunits) |
Low effectiveness of induction of the cellular link of immunity |
Prolonged and effective presentation of antigens to cells of the immune system (3–16) |
|
3. Joining of an antigen and an immune-stimulating carrier by a covalent bond |
Increase in reactogenicity of vaccine |
Decrease in molecular weight of antigen. Decrease in molecular weight of carrier. Use of a carrier that does not induce an immune response in the macroorganism. Elimination of the carrier and increase in antigenic density of epitopes of low-immunogenic peptides by a different method [14] |
|
|
Induction of an immune response to the carrier |
Conjugation of antigen with nonprotein carriers capable of hydrophobic interaction with cells of the immune system |
|
|
Presence of toxic components in the conjugates |
Replacement of methods using reductive amination |
|
4. Incorporation of antigen into initial complex |
Low immunogenicity of the complex |
Increase in size of complexes to 35 run on account of the incorporation of cholesterol and phospholipids |
|
|
Toxigenicity of the complex |
Purification of triterpene glycosides contained in the complex. Inclusion of lipids in the complex (5) |
|
5. Incorporation of antigen into lipid complex |
Low immunogenicity |
Replacement of lipids with aliphatic fatty acids by lipids containing long alkyl chains |
|
6. Incorporation of antigen into a "water—oil" or "oil—water" multiphase system |
Low immunogenicity |
Creation of rather small-sized drops (less than 1 /im), permitting macrophages to actively assimilate the composition as a whole. Incorporation of immunopotentiators as surfactants. Stabilization of the multiphase system. Incorporation of lymphokines into the composition (8) |
|
|
Toxicity |
Replacement of nonmetabolizable (mineral) oil by metabolizable oil (soy and peanut oils, squalene, etc.). Replacement of mycobacteria cells by complex compounds, for example, staphylococcal peptidoglycan, etc. |
|
7. Incorporation of antigen into a "liposome" type multiphase system |
Low immunogenicity |
Presentation of antigen on the outer surface of a liposome. Incorporation of components recognized by helper T lymphocytes or cytotoxic T lymphocytes into the liposome (9). Activation of division and differentiation of lymphocytes of the A and T subpopulations at the site of injection of the vaccine, inclusion of lymphokines in the liposome (8) |
|
8. Incorporation of lymphokines into the vaccine |
Low immunogenicity |
Stabilization of lymphokine in the vaccine composition. Production of fused genes of lymphokines and immunogenic proteins. Selection (construction) of lymphokine with definite properties |
|
|
Toxicity |
Combined administration of lymphokines with other immunostimulators in smaller doses. Addressed delivery of lymphokines, for example, in a liposome. Slow release of lymphokine from vaccine composition (11) |
|
9. Incorporation of structures that selectively interact with |
Low immunogenicity |
Construction of an antigen with structures capable of binding selectively to receptors on the surface of definite immunocompetent cells (tuftsin, LFA-3, etc.) |
|
10. Incorporation of genes of immunogenic determinants into viral vectors capable of persistence in the macroorganism |
Absence of infectiousness in purified viral DNA |
Construction of recombinant plasmids ensuring integration of foreign genetic material into the virus genome, etc. Improvement of methods of production of recombinant progeny. Changeover to DNA vaccines (15) |
|
|
Development of an infection process in weakened and immunodeficient patients |
Construction of expression vectors with low ability for replication. Changeover to bacterial exposure vectors (13). Changeover to DNA vaccines (16). Changeover to vaccines representing polymer compositions (11) |
|
|
Antigenic competition between the vector and antigen |
Enhancement of expression of antigen. Changeover to DNA vaccines (16). Changeover to vaccines representing polymer compositions (11) |
|
|
Development of complications after immunization |
Production of viruses possessing increased sensitivity to interferon. Cloning of genes of lymphokines into the genome of the vaccine-forming vector |
|
|
Low immunogenicity |
Use of vaccine-forming vectors that reproduce actively in the given organism. Effective expression of heterologous genes without substantial replication and persistence of the vector in the macroorganism (16) |
|
11. Incorporation of antigens into a polymer composition |
Low immunogenicity |
Use of polymers that lengthen the time of release of antigens from such compositions. Use of compounds whose decomposition products are immunostimulators as the polymers. Incorporation into polymer composition of: lymphokines (8); antigens bonded to an immunostimulating carrier by a covalent bond (3); antigens in the initial complex of lipid complex (4, 5); antigens incorporated into multiphase systems (17); antigens conjugated with antibodies (12). Changeover to DNA vaccines (16) |
|
12. Use of "antigen—antibody" complexes |
Low immunogenicity |
Increase in selectivity of Mabs interacting with immunocompetent cells. Use of antibodies for production of an immunosorbent, which is then used as an adjuvant capable of forming an antigenic depot |
|
13. Incorporation of antigenic proteins into surface structures of bacteria |
Low immunogenicity |
Exclusion of intracellular expression of antigen. Incorporation of antigens into proteins that form loop structures on the surface of the bacterial cell (LamB protein of E. coli, etc.). Synthesis of antigens in fimbria, lipoprotein signal polypeptide, and other systems permitting extraction of the antigenic protein into extracellular structures capable of hydrophobic interaction with immunocompetent cells |
|
|
Antigenic competition between vector and antigen |
Enhancement of expression of the gene of the antigen. Changeover to vaccines including polymer compositions (11). Changeover to DNA vaccines (16) |
|
14. Simulation of conformation of natural antigens |
Low immunogenicity |
For peptides that do not contain conformational epitopes — polymerization according to the "head-to-tail" principle or by the formation of intermolecular disulfide bonds between cysteine residues. Creation of complex conformational epitopes — cyclization of protein subunits and the use of morphogenetic assembly (structures of the "pseudovirus," "virosome" type, etc.). Adsorption (conjugation) on (with) microsphere carriers |
|
15. Incorporation of antigen into aluminum hydroxide gels |
Low immunogenicity Impossibility of induction of the cellular link of immunity |
Presentation of antigen to cells of the immune system by different methods (10, 13, 16) |
|
16. DNA immunization |
Low immunogenicity |
Enhancement and prolongation of expression of the gene of the antigen. Introduction of lymphokines together with plasmid DNA. Intracutaneous injection of DNA vaccine |
|
|
Development of complications after immunization |
DNA immunization is not used if the person was previously immunized with the given antigen by any other method |
|
17. Viral antigens |
Low immunogenicity |
Prolonged and effective presentation of antigen to cells of the immune system (3–16) |
|
18. Surface (envelope) viral proteins |
Toxicity |
Cloning (synthesis) of individual antigenic epitopes (see "narrow protective spectrum" and "antigenic drift") |
|
|
Narrow protective spectrum |
Cloning (synthesis) of conservative antigens (internal antigens) and conservative epitopes of surface antigens. Production of immunogens including combinations of conservative epitopes (antigens) |
|
|
Antigenic drift |
Incorporation of epitopes of internal structural proteins of the virus, as well as peptides encoded by regulatory genes, into the immunogen |
|
19. O-Antigens of bacteria |
Low immunogenicity |
Conjugation with carrier (3) |
|
|
Toxigenicity |
Removal of lipid A impurities |
|
|
Strain specificity of immune response |
Addition of outer membrane proteins to the composition (24). Creation of polyvalent vaccines (21). Changeover to other antigens (23, 24) |
|
|
Weak protection in the case of aerogenic infection |
Changeover to other antigens (24) |
|
20. Capsule polysaccharide of bacteria |
T-Independence of immune response Strain specificity of immune response |
Use of conjugates of polysaccharides with carriers in children up to 2 years of age (3) |
|
|
Strain specificity of immune response |
Creation of polyvalent vaccines (21). Changeover to other antigens (23, 24) |
|
|
Weak protection in the case of aerogenic infection |
Changeover to other antigens (24) |
|
21. Polyvalent conjugates of capsule polysaccharide and O-antigen |
High reactogenicity |
Changeover to other antigens (24) |
|
22. Flagellar (fimbrial) antigens of bacteria |
Strain specificity of immune response |
Changeover to other antigens (23, 24) |
|
|
Weak protection in aerogenic infection |
Changeover to other antigens (24). |
|
|
Instability of conformational epitopes to environmental factors |
Changeover to other antigens (19, 20, 23, 24) |
|
23. Lipid A |
Toxigenicity |
Production of less toxic analogs. Incorporation into compositions that slowly release the antigenic substance |
|
|
Low immunogenicity |
Conjugation with a carrier (3) |
|
|
Weak protection in aerogenic infection |
Changeover to other antigens (24) |
|
24. Porin proteins |
Formation of edema at the site of injection of porin |
Production of porin oligomers. DNA immunization with porin genes (16). Synthesis of peptides that stimulate antigenic epitopes of porins (14). Cloning of porin genes in bacterial vectors (13) |
|
25. Anatoxins |
Narrow protective spectrum |
Changeover to other antigens (23, 24). Use of combinations of anatoxins |
|
|
Toxicity of preparations |
Removal of endotoxin impurities that entered the preparation from the culture fluid |
|
|
Restoration of activity of toxin during storage |
Replacement of toxin inactivated by formaldehyde with genetically engineered detoxified derivatives of its subunits |
|
|
Low immunogenicity |
Prolonged and effective presentation of antigen to cells of the immune system (3–16) |
|
26. Antigenic complexes based on slime |
Toxicity |
Changeover to other antigens (24) |
|
|
Strain specificity of immune response |
Changeover to other antigens (24) |
If persistence of the pathogen of an infectious disease is accompanied by an antibody-dependent enhancement of the infection (individual pathogens of hemorrhagic fevers), then it is inadvisable to patent technical solutions that utilize this principle of functioning. During this decade, two externally unrelated approaches to counteracting such infections have been developed (Fig. 6). One proposes the creation of populations resistant to the pathogen, for example, including transgenic individuals that do not contain receptors recognized by the microorganism in the target cells. The other proposes elimination of the pathogen from the population by suppression of its replication and, consequently, of its transmission. Evidently in the future the two approaches will intersect, for example, in transplants of cells, tissues, and organs from transgenic individuals resistant to the pathogen to natural individuals. Figure 6 also shows that in the 1990s there has been a decrease in the number of alternative approaches permitting achievement of the blocking of replication of the pathogen within the framework of the technology of somatic gene therapy.
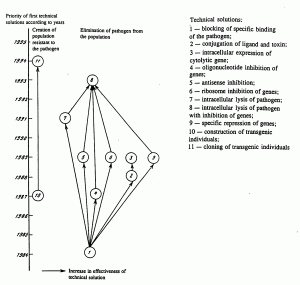 Fig. 6. Interrelationship of patentable technical solutions that exclude the immune response from the mechanism of protection from a pathogen of infectious disease.
Fig. 6. Interrelationship of patentable technical solutions that exclude the immune response from the mechanism of protection from a pathogen of infectious disease.
We should also mention that the effectiveness of most of the technical solutions created within its framework has been shown only in systems in vitro. Nonetheless, several requirements for the structure of constructs permitting intracellular inhibition of replication of the pathogen can be formulated:
1) for penetration into the somatic cell they should use a highly specific mechanism of receptor endocytosis;
2) blocking of replication of the pathogen should be based on highly specific recognition of its conservative structures by biological macromolecules synthesized intracellularly (regulatory peptides, ribozymes, antisense RNA, etc.);
3) induction of the synthesis of such biological macromolecules should be produced with products of the genes of the pathogen.
PATENT POLICY OF A BIOTECHNOLOGICAL ORGANIZATION
In the patenting of inventions, it must be considered that obtaining patents is not a goal in itself. Patents possess a dual nature. They not only provide the patent holder with the right to prevent the use of his or her invention but simultaneously permit targeted implementation of patent sanctions with respect to analogous developments of other persons, even if they were created by them quite independently. If the formula of the invention is cleverly put together, the volume of the patent may prove so great that even basic research will fall under the sanction, as was the case in the United States with investigations of cotton genetics after the patent of the California firm Monsanto was issued [7].
The most vulnerable with respect to patent sanctions is a product at the marketing stage [1]. Therefore, the appearance of patents is not random. By means of them the patent holder pursues definite goals. As a result, the competitive struggle acquires the "civilized forms" of patent policy.
The nature of the patent policy of a biotechnological organization will be determined by two main factors [11]: In the first place, patent protection may be offensive, anticipating possible changes on the consumer market, ensuring the closest to optimum protection of the biotechnological product being manufactured or proposed for manufacture; in the second place, it is produced in anticipation of expected claims on the part of stronger competitors, i.e., is of a. defensive nature.
The main methods that can be used by a biotechnological organization for implementing patent policy are shown in Table 4.
Table 4
Basic Methods Used in Implementing Patent Policy
|
Offensive patent protection |
Defensive patent protection |
|
Obtaining of blocks of patents preventing the penetration of rivals into the corresponding biotechnology field |
Claims for nonexisting solutions and solutions only noted in general outlines according to directions of activity of technologically stronger competitors (trap claims) |
|
Avalanche patenting in all directions of the activity of the rival firm |
|
|
Delaying legal processes for patents of competitors |
Expanded interpretation of the sphere of action of the protecting document using the theory of equivalents |
|
Obtaining patents on a "kind" of substances |
Selective patents on individual compounds of the "kind" |
|
Obtaining patents on preparations whose effect is based on a new, previously unstudied mechanism of action |
New use of a known substance |
|
Combination of claims for different objects into one formula |
Patents on long-action forms of known drugs |
|
Systematic patenting of each successive improvement of the object to be protected |
Patenting of drugs that can be used in a convenient and painless form |
|
Enforced licensing under the limit of misuse of patent monopoly |
Patents on technical solutions eliminating side effects of known drugs |
We should also mention that patentable objects are not equivalent from the standpoint of their "economic independence," i.e., the possibility of using an invention described in the patent without agreement of the holders of other patents. There is a definite hierarchy of patents [2,3]. Patents on chemical compounds axe first. As applied to objects of investigation, these are genes of antigens (or the antigens themselves), lectin-like compounds, ribozymes, antisense RNAs, etc. claimed as substances with an established structure (i.e., with an indicated nucleotide sequence for DNA and amino acid sequence for proteins). Such patents control the possible use of the technical solution; derivatives of the protected basic invention; other methods of preparation of the patented compound and new regions of its application; new compositions including the patented compound as a possible ingredient. Second are patents on known compounds and known mixtures of substances proposed for the first time for the treatment and prophylaxis of infectious diseases (for example, derivatives of the B-subunit of exotoxin A for the direct treatment of Bacillus pyocyaneus sepsis; EP 0261671), as well as patents on compositions (for example, a combined anthrax vaccine, containing spores of the anthrax microbe and a protective antigen; Russian patent 5060604). Methods of production of prophylactic preparations occupy the last (third) place in this "table of ranks" on account of the impossibility of controlling their use.
Thus, the nature and strategy of the patent policy of a biotechnological organization in the development of means of specific prophylaxis of infectious diseases can be determined on the basis of the technologies being developed by it and competing organizations, the limits whose framework bounds the development of each technology, and the patterns in the patenting of individual objects and peculiarities of pathogens.
REFERENCES
1. N.N. Karpova, Export Control and Patent-Licensing Policy of the Leading Capitalistic Countries [in Russian], Moscow, 1991.
2. A. Ya. Fogel, Patent Protection of Pharmaceutical, Inventions in the Capitalist Countries [in Russian], Moscow, 1988
3. I.Z. Mamiofa, Legal Protection of Inventions in the Capitalist and Developing Countries [in Russian], Moscow, 1986.
4. P.0. Gur'yanov, L.O. Sal'ts, and E.I. Furman, The Theory of Equivalents and Its Use in the Interpretation of the Patenting Formula [in Russian], Moscow, 1971.
5. Yu. I. Svyadosts, Bourgeois Patent Law [in Russian], Moscow, 1967.
6. S.P. Grishaev, Legal Protection of Inventions, Commercial Samples, and Useful Models in Russia and Abroad [in Russian], Moscow, 1993.
7. P. Ducur, Nature, vol. 387, no. 6629, pp. 13–14, 1997.
8. N.T. Rybal'skii and N.P. Shevelev, Objects of Biology and Biotechnology. Methodological Recommendations for Legal Protection [in Russian], Moscow, 1990.
9. N.G. Rybal'skii, Identification of the Model of Biological Objects and Development of Effective Approaches to the Protection of Biotechnological Inventions: Dissertation for the Degree of Doctor of Biological Sciences [in Russian], Moscow, 1990.
10. Methodological Recommendations for Conducting Patent Investigations [in Russian], Moscow, 1986.
11. R.B. Dauranov, "The patent service and the formation of patent policy of an organization," Voprosy Isobretatel'stva, no. 11, pp. 52–55. 1989.
12. L.V. Aleksandrov and N.P. Shepelev, Systems Analysis in the Creation and Development of Objects of Technology [in Russian], Moscow, 1992.
13. V.N. Tatochenko, N.A. Ozertskovskii, A.F. Sokolova, et al., Vaccine Prophylaxis: Handbook for Physicians [in Russian], V. K. Tatochenko and N. A. Ozertskovskii, Ed., Moscow, 1994.
14. B.P. Ivanov and A.B. Petrov, "Increasing the immunogenicity of peptide vaccines," Urgent Problems of the Creation and use of Immunobiological Preparations for the Diagnosis and Prophylaxis of Infectious Diseases [in Russian], vol. 1, pp. 35–53, 1993.
15. M.V. Supotnitskii, Vestnik Rossiiskoi Akademii Meditsinskikh Nauk, no. 8, pp. 18–22, 1996.
16. Patents 2178041, 18.03.83, GB, MKI C07K 3/00. Antigenic Modification of Peptides. Ohio State University, United States.
17. D.E. Hassett and J.T. Whitton, DNA Immunization, Trends Microbiol., vol. 4, no. 9, pp. 307–312, 1996.
18. H. Fu, W. Shen, and R.J. Collier, Vaccines 93. Mod. Approaches new Vaccines Includ. Prev. AID, New York, pp. 379–383, 1993.
19. Y. Singh, V. K. Chaudhary, and S. H. Leppla, J. Biol. Chem., vol. 264, no. 32, pp. 19103–19107,1989.
20. J. Angstrom, S. Neneberg, and K. Karlsson, Proc. Natl. Acad. Sci. USA, vol. 91, no. 25, pp. 11859–11863, 1994.
21. L. I. Romanova, "Preparations for emergency vaccine prophylaxis and diagnosis of rabies," Urgent Problems of the Creation and Use of Immunobiological Preparations for the Diagnosis and Prophylaxis of Infectious Diseases [in Russian], vol. 2, Perm, pp. 90–106, 1993.
22. Agrawal, D. Sun, P. Sarin, et al., J. Cell. Biochem., suppl. 14D, p. 145, 1990.
23. G. Scrakiel, M. Pawitta, A. Kleinheinz, et al., BGA Schriften, no. 1, pp. 263–264, 1990.
24. Patent 89/05852, November 10, 1987, PCT, MKI S12N 9/22. Ribozymes. Commonwealth Sci. and Industrial Res. Organization, Australia.
25. Patent 90/04414, October 18, 1988, PCT A61K39/395, Conjugates of Soluble T4 Proteins and Toxins, Biogen, Inc., USA.
26. L.K. Vencates, M.Q. Arens, T. Subramanian, et al., Proc. Natl. Acad. Sci. USA, vol. 87, no. 22, pp. 8746–8750, 1990.
27. Patent 93/06216, September 26, 1991. PCT MKI S12N 15/12, Fusion Proteins Targeted to Lysosomes, for Treatment of AIDS, Medical Res. Found., USA.
28. A.I. Polovinkin, Fundamentals of Engineering Creativity [in Russian], Moscow, 1988.
29. M.V. Supotnitskii, "AFTER AIDS... " Rossiiskii Khimicheskii Zhumal, vol. 11, no. 2, pp. 141–156, 1996.
30. A. Yu. Tsigankov, Novosti Nauki i Tekhniki, Seriya Biotekhnologiya, no. 9, pp. 31–35, 1989.
31. B.M. Mednikov, "Treatment of AIDS: problems and prospects," Priroda, no. 4, pp. 18–23, 1990.
32. Patent 2635532, FR, MKI C12N 15/51. Particular HBsAg Recombinant Hybrids, Inst. Pasteur, France, July 29, 1988,
33. V.M. Zhdanov, Epidemiology [in Russian], Medgiz, Moscow, 1961.
3.10.1997
Supotnitskii M. V. Effective Patenting of Agents For The Specific Prophylaxis of Infectious Diseases // Russian Biotechnology. – 1997. - № 10. – P. 15 – 35.

Михаил Васильевич Супотницкий
Российский микробиолог, полковник медицинской службы запаса, изобретатель, автор книг и статей по истории эпидемий чумы и других особо опасных инфекций, истории разработки и применения химического и биологического оружия. Заместитель главного редактора научно-практического журнала «Вестник войск РХБ защиты» Министерства обороны РФ.
Метки: 1997