After aids…
DICTIONARY OF TERMS USED
Vaccine — a preparation of a weakened or inactivated infectious agent (virus, bacteria, etc.) or individual components, carrying antigenic determinants, and able to produce formation of immunity (resistance) to a given infection in vaccinated objects.
Variolation — artificial infection with variola in order to produce a light form of the disease.
Vector — DNA molecule, capable of incorporation of foreign DNA and of autonomous replication, serving as the instrument for introduction of genetic information into the cell.
Virulence — a quantitative characteristic of the degree of pathogenicity of the microorganism.
Gene — segment of the DNA molecule, coding the structure of some cellular biopolymer (protein, RNA) or a certain regulator function.
Genome — the set of genes or chromosomes of the organism.
Integration — incorporation of individual fragments of a donor DNA into the chromosome (DNA) of the recipient cell.
Infection — process of incorporation of a pathogenic microorganism into the macroorganism or cells of a tissue culture.
Contagion — ability of infectious diseases to spread by transfer of the pathogen from sick people (animals) to healthy ones upon direct contact.
Cloning — the separation of mixtures of DNA molecules (e.g., recombinant plasmids, carrying in the composition of the vector molecule fragments of a foreign DNA) by inoculation on a nutrient agar of bacterial cells, into which DNA was introduced by transformation. One bacterial colony is a clone, all cells of which contain the same molecule of recombinant DNA.
Reverse transcriptase — an enzyme, the primary structure of which is coded in the genome of retro viruses; catalyzes the DNA synthesis reaction according to the RNA messenger using deoxynucleoside triphosphates as substrates and forming a polynucleotide chain, complementary to the messenger chain.
Plasmid — a round DNA molecule, autonomous from the chromosome, being replicated in the cell.
Prokaryotes — viruses, bacteria, which in contrast to eukaryotes, do not have nuclei.
Recombination — exchange of genetic material between two initial (parent) DNA molecules, leading to the formation of recombinant DNA molecules.
Reparation of DNA — synthesis of DNA at sites of damage of the DNAmolecule regenerating it to the initial state.
Retroviruses — RNA-containing viruses of animals, having in their composition an enzyme (revertase), copying RNA and DNA.
AIDS — acquired immune deficiency syndrome.
Transcription — synthesis of RNA on a DNA matrix.
Phenotype — property of an organism, dependent on its genotype and factors of the environment.
Eukaryotes — animals and plants, the cells of which, in contrast to prokaryotes, contain a nucleus with a membrane and chromosomes.
AIDS turned out to be a disease, very unexpected for the health care of industrially developed countries. The pandemic occurrence of AIDS revealed not only a discrepancy between the methodical level of conducted investigations and the complexity of the problems, which must be solved for liquidation of this disease, but also the amazing nearsightedness of prognoses of the ensuing epidemic situation, displayed in the first half of the past decade. Even in such a fundamental monograph as «Evolution of pathogens of infectious diseases,» written by the very well-known virologists V.M. Zhdanov and D.K. L'vov in 1984, virtually nothing is mentioned of the human immunodeficiency virus (HIV) [1].
In 1987 a group of authors under the direction of V.D. Belyakov, examining the problem of infections of the future, started from the fact that retroviruses are unable to give rise to an epidemic process, since their reproductive activity is not mediated by pathogenicity [2]. The reason for the inaccuracy of these prognoses lies in the fact that their creators based themselves on the statistical analysis of an epidemic situation of the last years and transferred it to subsequent years. For example, V.M. Zhdanov and A.G. Bukrinskaya, extrapolating the already modern epidemic situation, asserted that in the XXI century mankind will encounter such massive infectious diseases as influenza, herpes, hepatitis A and B, Dengue disease, and, it is understood, AIDS [3]. In addition, the possibility was not considered that in the future infectious processes, comparable in scale with pandemic diseases, can be caused by as yet undiscovered (or not appearing in the human population) pathogens.
An attempt is made in this paper on the basis of traditional biological concepts on the directivity of an evolution process, to look into the future of mankind, which can be characterized as «after AIDS»
1. BEFORE AIDS
Evolution of the Homo sapiens species occurred under the constant selective pressure of microorganisms, frequently producing very large-scale destructions in its individual populations. We do not know of the infectious pathogens, circulating among peoples of the prerecorded period of our history; however, the epidemic situation of at least the last 3000 years lends itself to documental analysis. Its main feature is the periodic change of pathogens, capable of pandemic distribution. Infectious diseases of various etiology came down on cities, countries, and even continents, causing a colossal loss of people, but were later forgotten by descendants of those who survived them. Thus, Hippocrates (IV century B.C.) writes nothing about smallpox, although we know from the history of the ancient culture of the Orient that variolation was known there even in the XII century B.C. [4]. Knowing the features of the epidemic extent of this disease, we find it difficult to imagine that, having originated in some country of the Mediterranean basin, it did not then penetrate into Greece. It can be proposed that smallpox was known on the European continent in the same years when variolation was in common practice in the Orient, but was then forgotten, and in the Middle Ages it appears already as a «new disease»
The plague, raging since ancient times, practically universally disappeared in the IX century. Its appearance in 1347 initially in the Azov Sea coastal region, and then in European countries, was also a surprise for contemporaries. And although it again disappeared from countries of Western Europe in the XVIII century, the preceding four centuries of the plague were, however, «a significant side of European history» [5].
Several severe and extensive epidemics of influenza occurred in the first half of the XIX century, but after 1850 this disease practically did not arise in a large portion of territories of the earth for almost 40 years (to 1886). And in 1918 the «returned» virus exterminated millions of people and very firmly fixed its position in human civilization [6].
The incidence of cases of smallpox began to decrease in the first half of the XIX century, which was exclusively attributed to immunization [4]. But the «Black Plague» in the XVII—XVIII centuries, unprecedented in its scales and aftereffects, arose in the presence of significant immune interlayers among the population of Europe. Consequently, the reason for the «disappearance» of a pandemic infectious disease is not compulsorily the formation of population immunity in the inhabitants. Only the abatement of the infectious process for a short period of time can be explained by the formation of immune populations, but its cessation for tens and even up to hundreds of years and subsequent return already require another explanation.
At the same time, the emergence in the human population of a new highly virulent and contagious pathogen is insufficient for occurrence of a new pandemic. Thus pathogens of Lassa, Marburg, and Ebola fevers are contagious and have repeatedly caused outbreaks of very severe illnesses, but not pandemics. HIV can in no way be grouped with highly virulent and contagious viruses.
The changes of pandemic diseases is also not explained by the theory of self-regulation of parasitic systems, presented by Belyakov and coauthors, who regarded «any pandemic process as self-regulation in the system of genetically and phenotypically heterogeneous parasite-host populations» [2]. A situation in which over several centuries with intervals of from 1 to 20 years (but not greater than the lifetime of one generation of people) the same pandemic process arises in the human community would correspond more to this theory. It explains well the epizooty and individual outbreaks of anthropozoontic diseases and the change of serotypes of their pathogens, but why highly virulent pathogens of the plague, smallpox, and influenza pandemics initially replace one another, and then unexpectedly give way to the slightly virulent HIV, cannot be understood on its basis.
On the level of subjective perception each new pandemic looks as if it considers the failures of the preceding one and at least by a step outstrips the possibilities of the knowledge of a given historical period. For example, smallpox and the plague, devastating Europe and Asia in the Middle Ages, could not be cured by the means, which medicine of this period had available. Both pandemics did not end by the rapid extermination of the Homo sapiens species only because their pathogens were recognized by the immune system of the majority of individuals, comprising it, and a few of the ill became less susceptible to the new infection. The extent of these pandemic processes with time is due to the fact that the massive death rate severed contact between infected populations and made it possible for populations to be formed of people, whose resistance to the plague and smallpox was already fixed at a genetic level [7,8]. However, the influenza A virus, spreading at the start of the XX century, «allowed for» the possible efforts of antiepidemic services on the creation of an artificial collective immunity and due to the constant change of its serotype was not controlled by it for more than a half century (until the appearance of effective vaccines).
Thus, it can be concluded that pandemics were accompanied by two processes, supplementing each other. One of them, the biological process, is displayed in the fact that the population of the Homo sapiens species periodically decreased upon the action of parasitic microorganisms, temporarily using the human populations as territories of their habitation and multiplication. Individual populations acquired the ability to recognize these microorganisms, to hinder their propagation, and subsequently to regenerate their number. Pathogens of such diseases cannot exist long historically inhuman populations. They either are displaced into another ecological niche, e.g., the plague pathogen, or are eliminated from them without formation of a new reservoir, which, in the opinion of many epidemiologists, also occurred with the smallpox pathogen [7,8].
An alternative to these processes was more frequently the sustenance of pathogens in limited populations of people as less harmful strains. In the XVI—XVIII centuries in many countries of Europe pockets of the plague and smallpox existed in parallel [5]. Such pockets «peacefully got along» in Southwest Asia up until the middle of the XX century [4,6,9]. The circumstance that they were formed not by pandemic strains, agitated them to reduce the territory of habitation of one in the other.
The social process appeared in the constant accumulation of possibilities of human society for active action on the distribution and propagation of pathogenic microorganisms. The number of Homo sapiens populations increased significantly as a result. The equilibrium observed in nature for millions of years, between individual species from the Hominide group and their parasites and also between the parasites themselves, competing for a set habitat and mutually regulating the density of their populations, was disturbed.
The disturbed biological equilibrium is regenerated most frequently at some other level. The question is how is this displayed. Mass immunization and antibiotic (chemo)therapy sharply narrowed the possible region of habitation of parasitic species to single recipient persons, not immunized for some reason or not having undergone courses of nonspecific medical treatment (prophylaxis) with antibiotics or chemical preparations. The possibility to recognize pathogens of an infectious disease, to carry out quarantine measures, limiting contact of diseased persons with the well persons, and other actions, hindering the spread of pathogens in the environment (disinfection, decontamination, etc.) create a situation, similar to that which occurred before formation of large settlements of people (and this during the gregarious existence of their ape-like predecessors), when infected persons lived on widespread territories and, becoming ill, could not interact with one another for a long time. Therefore, the appearance in mankind of a community of pathogens, capable of pandemic distribution at a low population density, is an objective regularity.
At the same time it should be recognized that the described situation is quite arbitrary under modern conditions. Extraordinarily dense populations of the Homo sapiens species today enter into relations with microorganisms of the «host-parasite» type. They simultaneously interact with no less dense populations of domestic animals, birds, rodents, insects, and their microorganism parasites. The latter, if they can expand the territory of their habitat, having penetrated into some large population of the Homo sapiens species, can be recognized there and can exist under conditions, when their interaction with individual representatives of the population will be sharply restricted. Hence the trend in the selective screening of signs of parasites, new for immunocompetent persons of the Homo sapiens species — moderate virulence, prolonged incubation period and carrier state, the absence of characteristic symptoms in the infectious diseases, caused by them, resistance to antibiotic and chemotherapeutic agents, the impossibility of control with the use of immunoprophylaxis agents, susceptibility of those who had been repeatedly infected with these same pathogens, transmission pathways, excluding control of the distribution of pathogens with the use of restrictive measures and actions in the environment.
2. AIDS
2.1. Characteristics of the pandemic process
The AIDS pandemic, appearing in the human population at the beginning of the 1970s, clearly outstrips the resources of knowledge at the end of the XX century. Effective quarantine measures are not possible for this illness due to the long incubation period. The development of any agents and methods of medical treatment and prophylaxis ran into the absence of the corresponding experimental models. It should be recognized that the resources of modern medicine in relation to HIV are less than those which were available in the medicine of the XIV century for the plague pathogen and in the XVII—XVIII centuries for smallpox. At the same time, the pandemic process itself, despite a sluggish course, has a more dangerous character than do pandemics of smallpox or the plague, since a mechanism of effective self-restriction to the spread of HIV is absent in nature.
This position requires a more thorough examination. The author would like to warn against analogies with other infectious processes and primarily with such a popular example as the spread of myxomatosis among rabbits in Australia. This altogether special case N. Ampel [7] even extrapolates to the whole history of the interaction of pathogenic microorganisms with populations of their hosts.
The Ampel hypothesis, constructed on the analysis of this case, is simple. The highly virulent pathogen penetrates into the population of the new host and produces a devastating epidemic; however, it loses virulence during this, since chains of transfer of the more virulent strains rapidly are broken. Selection of the more resistant species of the new microorganism-parasite host occurs simultaneously. The hypothesis is difficult to contest; it is competent, but only in relation to pathogens of fast infections, e.g., the plague and smallpox, leaving after itself some number of immune specimens. However, to what extent is it applicable to AIDS?
First of all, we note that the situation with Australian rabbits was not as schematic as this is seen by Ampel [7]. At the first stage of the experiment, i.e., until the myxomatosis virus stopped eliminating rabbits «always less and less,» it in fact destroyed 99.5% of their initial population [6]. However, in this region the myxomatosis virus was transmitted by mosquitoes of the Culex annulirostris species [6]. The remaining living (i.e., resistant to myxomatosis) rabbits regenerated the number of their populations during the winter months, when there were significantly fewer rabbits, and consequently, less virus [6]. In England, where the transfer agents of the myxomatosis virus were fleas, the virulence of pathogen strains practically did not decrease, and the genetic resistance of rabbits to the virus did not occur in the 12 years of observation [6]. This situation is explained as follows: «mutual exchange» of fleas occurs for three days between healthy and diseased rabbits under natural conditions; the length of the disease is 11–18 days. In addition, after the death of animals the infected fleas can remain in the burrows up to 105 days without losing their infectiousness. In other words, a high density of diseased animals in their populations was not required for infection of one animal. It was sufficient that it «drop» into the empty burrow once in 3.5 months. The retention of an insignificant portion of wild rabbits in England was associated with the change in their mode of life. They left ancestral burrows and began to live on the earth's surface [6]. However, if infection by myxomatosis were achieved by a sexual path (like HIV), then during such sexual activity of the species and the rate of development of the disease, the formation of rabbit populations, resistant to the virus, would be out of the question.
Thus, on the basis of the analysis of this, almost chrestomathic episode prognoses cannot be constructed in relation to the outcome of the HIV pandemic. The inevitability of the joint evolution (coevolution) of any parasitic forms of microorganisms and their new hosts also cannot be proposed.
The situation is intensified for the Homo sapiens species because HIV causes a disease, the spread of which does not require a high population density, sustaining the continuity of the epidemic, as this occurs in the case of the spread of the plague, smallpox, or influenza. The lengthiness of the epidemic process with time in this case does not establish a stable human population, but increases its infectiousness. The length of the carrier state and the absence of steady postinfection immunity compensate for the low infectiousness of the virus itself and the absence of its massive release into the environment with the infection of a large number of people. The diseased not only are not taken out of the population, but as a result of mental disturbances cannot critically accept their state [18,45]. The uninfected portion of the population because of legislative restrictions cannot avoid contacts with such diseased persons and completely depends on their «good will.» Children, born to HIV-infected mothers, as a rule, are also infected [45]. The palliative character of medical treatment of patients with AIDS also leads to an increase in infection of the population. The prolonging of their life without the possibility of a total cure and due to prolonging of the incubation and symptomatic periods of the disease have lead to the case that the rate of spread of the pathogen increasingly outstrips the death rate from it [28].
In addition, it is now clear that HIV-1 and HIV-2 are members of a large group of immunodeficiency retroviruses, infecting various species of primates [10]. It is not excluded that unknown forms of HIV have also penetrated the human population. Thus, the most attention at the 8th International Conference on the Problem of AIDS was given to reports on patients with progressing immunodeficiency upon the absence in them of HIV-1 and HIV-2. It is considered that a new virus is transmitted more easily than the virus producing AIDS [11]. There is a communication that the reason for an autoimmune disease of humans, called Sjogren's syndrome, is an infection with a retrovirus, antigenically similar to HIV, but containing functionally differing reverse transcriptase [29].
Are these diseases random? We will attempt to answer this question by examining regularities and trends, displayed in the last century in the evolution of pandemic viruses.
2.2. AIDS and similar diseases as the manifestation of a definite regularity in evolution of pandemic viruses
Data on the viruses, causing the appearance of infectious diseases, «new» for people of the XX century, are presented in Table 1. They all have constant reservoirs in nature. Theoretically any of them can rapidly mutate, but according to Holland, RNA viruses have a higher frequency of mutations than do viruses with DNA genomes of the same size [13]. This is normally explained by the absence of correction mechanisms in the synthesis of RNA. Thus, the more probable appearance of a new pandemic RNA, and not DNA virus (first regularity) can be regarded as a definite regularity.
Table 1
Examples of Development of «New» Viruses in the XX Century
|
Virus (genome type) |
Symptoms |
Distribution |
Natural host |
|
BUN'YA VIRUSES (RNA) |
|||
|
Hantaan, Seoul, etc. |
Hemorrhagic fever with kidney syndrome |
Asia, Europe, USA |
Rodents (e.g. of the Arodemus genus) |
|
Pathogen of Rift Valley fever* |
Fever can also cause hemorrhage |
Africa |
Mosquitoes, Ungulata |
|
Oropouche* |
Fever |
Brazil, Panama, Trinidad |
Two-winged insects |
|
TOGAVIRUSES (alpha RNA) |
|||
|
O'N'ong-n'ong* |
Arthritis, rash |
Africa |
Mosquitoes |
|
Sindbis* |
The same |
Africa, Europe, Asia, Australia |
Mosquitoes, birds |
|
FLAVOVIRUSES (RNA) |
|||
|
Rossio* |
Encephalitis |
Brazil |
Mosquitoes, birds |
|
K'yansanursk forest disease |
The same |
India |
Ticks and mites, rodents |
|
ARENA VIRUSES (RNA) |
|||
|
Junin (Argentinean hemorrhagic fever) |
Fever, hemorrhage |
South America |
Calomys musculinus |
|
Machupo (Bolivian hemorrhagic fever |
The same |
South America |
Calomys callosus |
|
PHYLOVIRUSES (RNA) |
|||
|
Marburg, Ebola |
Fever, hemorrhage' |
Africa |
Unknown |
|
LENTIVIRUSES (RNA) |
|||
|
Human immunodeficiency virus |
AIDS, set of symptoms, characteristic for AIDS |
Over the whole world |
Primates (?) |
|
POXVIRUSES (DNA) |
|||
|
Primate pox |
Characteristic for smallpox |
Africa (wet jungles) |
Squirrels |
Note. Viruses transmitted by Anthropoda are indicated by the symbol * [12].
Analysis of the rate of development of rapidly evolving viruses «shows that their surface structures change significantly more rapidly than do their internal structures.» For example, Doolittle and coworkers [14], studying the rate of evolution of 10 genes of retroviruses, noticed that the fewest changes are observed in the gene of reverse transcriptase, and the greatest changes are observed in genes, coding surface proteins (by 3 times). The internal portions of the protein gag changed approximately 1.5 times more rapidly than of transcriptase and 1.8 times faster than of protease. From this it can be concluded that determinants of tissue tropism change primarily — this is the second regularity in formation of new pandemic viral pathogens.
Analysis of the tropism of the most significant pandemic viruses shows that these changes are pointed in the direction of the appearance of a specific effect of the virus. For example, the smallpox virus infects epithelial tissues, the influenza virus infects the surface epithelium of small bronchi, and HIV infects cells and tissues, having the SD4 receptor. Consequently, the specialization of the infecting effect is also a type of regularity (the third) in the evolution of pandemic viruses.
Selection of mutants is not the only possibility of formation of new pathogens from viruses with nonsegmented genomes. Analysis of genetic sequences showed the virus of Western horse encephalomyelitis arose evidently 100–120 years ago as a result of recombination, in which a Syndbis-like virus and a virus of Western horse encephalomyelitis participated [12]. The Rossio encephalitis virus could have arisen similarly. Genetic recombinations evidently occurred between genes of membrane proteins of T-lymphocytic HTLV-1 and HTLV-II human viruses [12]. A definite regularity is also observed here (the fourth) — new viruses are formed as a result of their recombination if they use the same transmitting agent or host. This regularity upon a knowledge of the trend in selective pressure on the virus population makes it possible to foresee the appearance of a definite set (combination) of genes in a new recombinant.
Thus, it can be concluded that a new pandemic virus will undoubtedly have a RNA genome and will possess tissue tropism, different from preceding pandemic viruses, and a higher specialization of infecting effect. The development of these properties will become possible due to mutation changes in known or, on the other hand, «unknown» viruses, or as a result of recombination of viruses of one natural reservoir. Let us examine what groups of RNA viruses evolve more rapidly than do others under the present conditions and thus narrow the circle of our searches.
According to data of specialists of the Los Alamos National Laboratory (USA), namely HIV at the present time possesses the greatest mutability [16]. The mutation processes in it occur 10 times more rapidly than in the influenza virus and 100 times more rapidly than in cellular DNA. Several viral subtypes can be present simultaneously in the organism of patients [3]. A hypothesis has been presented that HIV-1 and HIV-2 «circulated» approximately 40 years ago, and that the occurrence of the pandemic in the middle of the 1970s was due to an increased rate of mutation of the virus in the 1950s [16].
However, the high level of HIV morbidity of the population to the south of the Sahara made it possible for R. Anderson and R. Mei to propose that HIV has existed there for a very long time [28]. From results of the analysis of the molecular structure of the virus they estimate this period 100–200 years and more [28]. But why did the HIV pandemic not occur earlier, e.g., in the time when slavers transported millions of people from Africa? Being given this problem, we unavoidably come to the hypothesis that some powerful factor existed, hindering the spread of HIV for a very long historical period. What promoted the acceleration of mutations and spread of the virus in the 1950s, particularly in the 1960—70 s of the XX century?
The beginning of both processes with time clearly coincides with other phenomena, namely with the sharp decrease in the incidence of cases of smallpox in the population of a region [4]. In contrast to the spread of smallpox and HIV in Northern Africa, Europe, and on the American continent, these diseases were not endemic in countries south of the Sahara, but were endemic [28,56]. The population of namely those countries, where now HIV is universally widespread [28], was infected until the start of the 1950s with the smallpox virus to a significantly greater degree than in other regions of Africa [4,56]. Under these conditions people, infected with AIDS, could not exist very long. The endemic smallpox virus broke the chain, along which HIV, endemic in a given region, spread. As a result HIV did not have the possibility to evolve, and its variants, preserved in more ancient hominids, in no way appeared in people on the background of other mass infections.
Antivariolous immunization, long carried out by the scarification method in regions, endemic with respect to both pathogens, and the subsequent decrease in the number of cases of smallpox in the population made possible not only the prolonging of the life of people, ill with AIDS, but also increased the layers of the population, infected with HIV. While the Homo sapiens species earlier had been for HIV nothing more than an outer segment of the habitation zone of retroviruses of other primates, it now became a new ecological niche for it. The absence of selective pressure on the pathogen with a constant expansion of its zone as a result of an increase in the number of the human population in the endemic region, and also the expulsion in developed countries with the use of antibiotic (chemo)therapy of other possible HIV competitors — pathogens of fast infections, favored the selection of quasiforms of the virus, causing the AIDS pandemic at the end of the XX century.
A careful analysis of the structure of the infectious sick rate at the beginning of the 1990s indicates the existence of not one, as this is normally considered, but of at least two global epidemic processes, developing in parallel, due to retroviruses. Thus, P.V. Markarov and coauthors, analyzing the modern stage of evolution of the global epizootic situation, noted the presence for a trend in the spread among sheep of the visna-medi diseases, caused by retroviruses, representatives of the same subgroup as HIV, i.e., lentiviruses [17]. The reasons, causing the appearance of the disease were not determined, but it is known that it first appeared in 1933 in Iceland [6]. A.G. Bukrinskaya and V.M. Zhdanov proposed on the basis of the analysis of the homology of nucleic acids of both viruses that HIV and the visna virus have a common ancestor [3]. There are as yet no convincing data on the danger of the latter to man, but the similarity in the clinical course of visna in sheep and of AIDS in humans is surprising [18]. N.A. Dobrotina even presented a hypothesis, explaining the AIDS epidemic in Elista in 1987 by the penetration of the visna virus into the blood of people upon the slaughter and burial of millions of fine-wooled sheep, dying from it in the winter of that year in Kalmykiya [46]. Although another explanation was subsequently presented for the reasons for the epidemic, the possibility of infection of people by the visna virus should be considered seriously. Thus, the analysis of regularities of the appearance of new viral pathogens, the trends in their evolution, and also the global epizootic situation indicates a sharp increase in the epidemic significance at the end of the XX century not only of HIV, but also of viruses of this group as a whole.
3. AFTER AIDS
Let us explain what situation we recognize as «after AIDS.»
1. HIV is only one of the representatives of lentiviruses, causing extensive epidemic processes in the XX century in populations of people and domestic animals. Competition is also possible between representatives of this group of viruses for the territory of its habitation, as a result of which only the most adapted form remains in this ecological niche (in evolution biology this process is called the search for the optimal phenotype [19]). In addition, competition is possible between retroviruses and other mobile «inhabitants» of primate chromosomes. It is considered that approximately 0.1% of their genome consists of virus-like sequences [47].
2. The spread of AIDS, leukoses, and serum hepatitis, and the wide use in clinical practice of cytostatics and immunodepressants leads to the gradual decrease in immune protection in millions of representatives of the Homo sapiens species, living densely, and creates conditions for the penetration and then its «population» with other microorganisms, which will have the indications of an infectious process. In addition, the selection and formation of epidemic variants among microorganisms, not considered earlier as such, is significantly facilitated under these conditions. Therefore, upon attainment by the immunodeficient populations of a certain threshold level or their merging into one quite large (global?) population the formation of epidemics is very possible, caused by «overcome»and «controlled» microorganisms, their closely related forms, and also pathogens, extremely unexpected for health services of developed countries.
3. Agents of immunoprophylaxis and antibiotic and chemotherapy, widely used in the XX century, are important components of global selective pressure on parasite microorganisms. Achieving (in a short perspective)victories over individual infectious diseases, they simultaneously create conditions for the evolution selection of species, becoming parasites under new conditions. The possibilities of technologies, used for the further improvement of such agents, are not unlimited and will unavoidably be exhausted. The creation in their framework of new therapeutic and prophylactic preparations, making it possible to restrain the development of epidemic processes launched by them, will become impossible as a result.
3.1. Pathogens, able to displace HIV
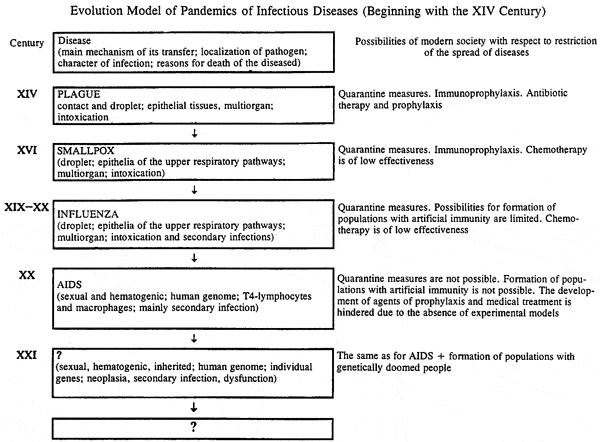
Demonstrations of the regular penetration of HIV into the human population were presented in the preceding section. Of significantly great interest at the present time is the pathogen of the next pandemic — when and under what circumstances it will arise, the possible aftereffects of its spread. In order to come to an understanding of this, let us examine the internal regularities of a change of pandemic processes. The evolution model, presented in Scheme 1, shows that each pandemic process, occurring in the last six centuries, did not resemble the preceding ones and was not repeated in subsequent ones. What occurred in this relatively short historical period?
Scheme 1
First, the bacterial pathogen of the pandemic (the plague) was initially replaced by a virus with a DNA genome (smallpox) and then by viruses with RNA genomes (influenza, AIDS). This process was accompanied by a change in mechanism of their transfer. Contact and droplet (plague) mechanisms were displaced by droplet (smallpox, influenza) and then by sexual and hematogenic mechanisms, significantly less dependent on the effect of the external medium (HIV). The targets and specificity of the infecting effect of pandemic pathogens change simultaneously. Highly virulent pathogens of pandemic diseases (the plague and smallpox) are replaced by less virulent (influenza) and slightly virulent (HIV) pathogens.
Second, during the replacement of pandemic pathogens, despite all launched efforts, the objective possibilities of human society for restriction of their spread to the natural population decreased consecutively. At the end of the XX century the most «conquered» appears to be the plague. The achievement of quarantine measures, immunoprophylaxis, nonspecific emergency prophylaxis, and the medical treatment with antibacterial preparations led to ejection of the plague from the human population and to the parallel existence (and conservation) in another ecological niche. This smallpox virus is considered «conquered,» although vaccine prophylaxis and quarantine measures remain to date the only means for prevention of its spread in the human society. The possibilities of isolation measures, vaccine prophylaxis, and chemotherapy for control of influenza are significantly limited. With respect to HIV, then mechanisms generally do not exist for the self-restriction of its spread in populations of peoples.
Third, the trend in global selective pressure on microorganisms — parasites increasing at the end of the XX century, promotes the selection and pandemic spread of those of the species, which preserve themselves by integration into the human genome.
Inherent logic and the succession of events, presented in the evolution model (Scheme 1), make it possible to propose that the next pandemic (after AIDS) will be due to a microorganism, which not only is incorporated into the germe and becomes a genetic parasite [3], but also actively destroys its segments or individual genes, important for the vital activity of man (Table 2).
Table 2
Possible Properties of a Pathogen, Able to Displace HIV
|
Character of the infection process (in comparison with HIV) |
Biological significance of evolution of the pathogen |
Social factors, promoting the appearance of the pathogen |
|
In addition to sexual and hematogenic, the main mechanism of transfer of the pathogen will become hereditary |
The pathogen discovers «immortality» and will exist as long as the Homo sapiens species |
Prophylactic measures, associated with early detection of AIDS |
|
Increase in possibilities of integration at various segments of the human genome and also the number of integration sites |
Expansion of the characteristic ecological niche |
The use of gene-therapy technology for the medical treatment and prophylaxis of AIDS |
|
Disturbance of regulation of metabolic processes, neoplastic changes, and other pathology associated with intervention in the work of the human genome |
Retention in the pathogen of the ability for persistence under conditions of counteraction to the reparation and other similar systems of the host, regarding it as a harmful mutation |
Retention of genetically defective (infected) persons, maximum prolongation of the duration of their life with the possibility of reproduction |
The ability of viruses to destroy genes upon integration into the genome of eukaryotes is an already known phenomenon [15]. However, in this situation this process will be a result of a competitive battle of retroviruses for the effect on the genome both among themselves and with those mobile elements of chromosomes, which possibly play the role of «protective shields» of the genome from such viruses. That microorganism which is capable of more effective integration and counteraction against the protecting systems of the host will have an advantage. An increase in the number of integration sites, blocking of repair systems of the macroorganism, competition with HIV gene-transactivators, relocation with respect to the genome with capture of DNA segments, and integration into homeosis genes (regulator genes of higher order, controlling the work of other genes) will lead to the formation of a pathology, which will far from immediately be perceived as infectious.
The successful battle against AIDS will help promote the spread to the human population of such a disease, which at first glance will not appear unusual. The possibility of onset of following events is evident here.
Considering that HIV is integrated into the human genome and as it becomes its natural operon, we can propose that namely in the framework of technologies used in gene therapy, can effective approaches be developed to the medical treatment and prophylaxis of AIDS. But when such segments of the human genome, being sites for integration of HIV, or the genome of the virus itself becomes target for gene therapists, conditions arise for the direct selective pressure on the population of retroviruses. In their turn, retroviruses are not uniform and contain different variants of even the same pathogens. From these variants, «extruded» from segments of the genome, traditional for them, can begin the formation of pathogens, integrating into segments of the genome, which were inaccessible for a certain time to gene therapy, e.g., in homeosis genes. Disturbance of the function of the latter is equivalent to disturbance of large segments of the genome. The appearance is possible of retroviruses, not having definite integration sites or (the worst variant for a given situation) not displayed in the first generation of their new hosts.
Of existing pathogens such a pandemic after certain mutation can be caused by a little-known (or as yet unknown) variety of oncoviruses, belonging to the group of retroviruses and somehow falling into the human population from the animal reservoir (e.g., as a result of experiments on organ and tissue transplants, the use of vaccines, prepared from animal cell cultures, skotolozhestva, etc.) or formed as a result of recombination of viruses, utilizing one ecological niche (e.g., having common integration sites in the genome) and for the above-indicated reasons receiving selective preference over HIV.
With respect to features of a new pandemic situation, it would be desirable to turn our attention to the fact that integration of HIV into the gene does not occur without leaving a trace on mankind. Already during the first description of this disease a high mortality of patients from tumors was noted, particularly from cancer of endothelium, which serves to some degree as an indirect confirmation of the selective infection by HIV of individual human genes [18]. There are also direct experimental confirmations of this. It was shown that the introduction into transgenic mice of the HIV regulator gene tat causes in them a pathological state, reminiscent of Kaposi's sarcoma [3]. It can be concluded that the process of formation of such a pathogen had already begun in the subgroup of lentiviruses, although in the disease produced by their form, most widespread at the present time, has extremely different pathogenesis.
The clinical manifestations of a new disease can be quite difficult to describe. They can be rapidly progressing neoplasms, immunodeficiencies, muscular dystrophy, demyelinization, accumulation of amyloid proteins, mental disturbances, defects in development, and other infections of genetic character. However, if we start from the fact that a new pathogen is able to utilize ecological niches of HIV, dislodged by them, then the most probable is a pathology, associated with DNA defects of T-lymphocytes, endotheliocytes of blood and lymphatic vessels, epithelial cells of the skin, astrocytes and neurons of the brain.
The clinical manifestations of the diseases can be reminiscent of AIDS to infectionists. But they cannot confirm the diagnosis by observation of antibodies to HIV-1 or HIV-2 and cannot attain an improvement in the state of the patient by the use of azidothymidine. The incubation period evidently will exceed that of AIDS. An additional transfer mechanism can be added — hereditary.
Thus, it can be concluded that a new model of a pandemic process is formed, when the pathogen can be transmitted without entering the environment. Such a path is not new for higher animals. It is assumed that the gross virus of mouse leukosis and the Bitner agent of carcinoma of milk glands is transmitted by the genetic pathway from mother to progeny [3]. Continuance of the pandemic process for several decades and, completely probably, for up to centuries, and the partial erasure of differences between pathologies of inherited and infectious viruses upon the active use of gene-therapy technologies and formation of transgenic populations of people, are hence possible.
It can be assumed that the formation of vast populations of genetically doomed people will become a remote result of the pandemic spread of disease. The answer to the question of why this has not occurred to date can be found if the «development tree» of hominids is analyzed (Scheme 2 [20]). Then the different sensitivity of man and man-like primates to HIV and other lentiviruses becomes understandable [18]. From the late Myocene, i.e., 10 million years ago, these species of primates developed independently; each had its history of interaction with specific retroviruses. Not only mutual adaptation, but also rigid selection of both biological species — viruses and macroorganisms — occurred simultaneously [10]. This process did not cease in the XX century.
Scheme 2
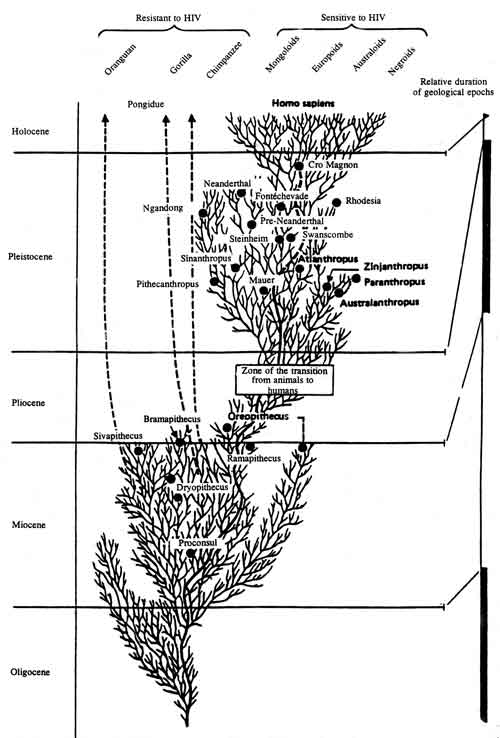
Yu. G. Matushkin and S. N. Rodin investigated by the method of mathematical modeling the possibility of existence of homeostases of the «genome-genetic parasite» biosystem under various conditions. They showed that if the genetic parasite is incorporated into some segment of the genome, significant for the vital activity of the organism, then the organism dies. In this case the question reduces to whether a genetic parasite of such type will succeed in entering the majority of genomes of the Homo sapiens species [49]. In contrast to other endogenic transposition elements of the human genome (e.g., retrotransposons), retroviruses are capable of replication in the cytoplasma. Therefore, they multiply more rapidly than does the host population and consequently, upon the presence of a transfer mechanism are able to infect it all during some number of generations (the length of this process does not have any significance from an evolution point of view).
The presented data simultaneously indicate the possibility of existence of a mechanism of random (i.e., not due to a sharp deterioration of external conditions of its habitat) multiplication of the species, acting on the genetic level. Retroviruses of the HIV type or those hypothetic oncoviruses from the same species, which as yet «follow» AIDS, can «trigger» the mechanism; however, the determining role in this process is probably played by new pathogens, appearing in populations, infected by retroviruses. For the Homo sapiens species at the beginning of the XXI century they can be low-pathogenic pathogens of various nature, not controlled by immunoprophylaxis and antibiotic and chemotherapy measures.
The dying away of one species during coevolution processes does not mean the disappearance of the whole group (e.g., primates, not considered extinct species, consist of four suborders, 11 families, and 60 genuses [50].) This can hold true to an identical degree for both HIV and its new host — the Homo sapiens species, and for those species, which continue their coexistence further. The Nobel Prize laureate J. Lederberg, appearing at the conference «New Viruses: Evolution of Viruses and Viral Disease (Washington, May 1–3, 1989),» noted that the only real competitors to humans for domination of the planet are viruses, which can fulfill the role of both parasites and of genetic elements [12].
3.2. «NEW» PATHOGENIC MICROORGANISMS AND THEIR TOXINS
The analysis, carried out by Ampel, of outbreaks of new infections diseases (Lyme disease, Legionnaires' disease, toxic shock syndrome, and AIDS) shows that although these diseases appeared unexpectedly, their individual cases were observed before the development of the present epidemic [7]. In addition, it was observed that all new diseases are due to pathogens, existing earlier in natural reservoirs, but they either were not recognized or they were not taken seriously.
The trend and stages of evolution of pathogens of infectious diseases have been examined by many domestic and foreign epidemiologists [1,2,9,14,21,22]. Data on the relatively recently forgotten microorganisms and their toxins, which either have phenotypical indications, providing them during existing technologies of control of infectious diseases with advantages over their predecessors (closely related forms, appearing as pathogens of infectious diseases of humans), or possessing pathogenic potential, occupy that ecological niche, from which they can easily penetrate into the human population, are presented in this section.
Smallpox. The disease pathogen, a representative of a very numerous family of poxviruses, was still recently widespread on all continents and carried away millions of people. Smallpox is now considered a «conquered disease» [8]. However, when claims are made for the eradication of some pathogen of an infectious disease, the circumstance is not additionally evaluated that interaction between parasites and their hosts occurs not only at the level of biological species, but also in their families. The ability for adaptation in families is incomparably higher than in any of its individual species [19].
Data are presented in papers of Baxby [8] and Zhdanov [51] on at least three varieties of viruses of a given family, sustained at the present time in natural reservoirs and able to produce infectious processes in people (Table 3).
Table 3
Features of Poxviruses from Natural Reservoirs
|
Virus |
Features of the disease |
Contagiousness |
Natural reservoir |
|
Monkey smallpox virus |
Clinical picture is practically the same as for smallpox. Mainly infects children and unvaccinated people. Fatal results are possible |
Transfer upon contact is possible. Up to 12% of unvaccinated people, coming in contact with the diseased, become ill |
African continent Apes, squirrels |
|
Tree shrew pox virus |
The course is easier than during monkey pox. Differentiation between these two diseases is difficult from clinical symptoms |
Spread of the infection from humans to humans is encountered extraordinarily rarely, but it is nevertheless possible |
African continent Nonhumanoid primates |
|
Cow pox virus |
Encountered in people and domestic cats, Course most frequently light. The virus possesses low infectiousness for humans |
Diseases are known among people, getting the infection as a result of indirect infection, e.g. upon contact with barbed wire, blackberry thorns, etc |
Great Britain Voles, field mice |
In this connection, very interesting is the observation of high conservatism of nucleotide sequences in central regions of genomes of orthopoxviruses. The differences are concentrated mainly in the vicinity of ends of DNA molecules. Terminal fragments of the genome are hypervariable; large deletions and complex reconstructions of genetic material are observed in them [15]. Evidently isolation of individual species in different ecological niches occurred long before the appearance of the smallpox virus as a result of evolution divergence in the Poxviridae family. This removed competition between subtypes and led to the formation of types, not competing among themselves, upon relatively small genotypical differentiation between them. Therefore, representatives of the Orthopoxvirus genus, «donating» the smallpox pathogen to humans, differ among themselves in minor sequence of genomes, changing only those which participate in preservation of their populations under constantly changing conditions in the habitation medium.
Specialists on orthopoxviruses have already for two decades been strongly convinced of their victory over smallpox [8]. From evolution positions their victory over smallpox is only the displacement of the species, possessing a nonadaptive and extreme phenotype. It can be expected that upon formation of large immunodeficient populations in the Homo sapiens species conditions will appear initially for «passivating,» and then for return to the human civilization of representatives of this family. The high density of populations and the actually complete disappearance of pox-immune layers, formed by centuries, will favor this process. Epidemiologists should be alerted by the circumstance that the largest immunodeficient population of peoples was formed namely in those regions of Africa, which earlier were endemic in relation to smallpox; natural sources of orthopox viruses still were retained in their, able to cause contagious diseases in people [8,28,56].
Modern control of the smallpox pathogen cannot be considered reliable. WHO considers that the last endemic case of this disease was detected in 1977 in Somalia [8]. However, very clear evidence was published by L. B. Shebarshin on a case of pox in Kabul in 1982. It can be considered more a memoir than scientific, but what does that change [24]? The scientific literature contains indications that countries, reporting cases of smallpox to WHO, did not know of a real epidemic situation in a series of their regions. An outbreak of smallpox, occurring in 1962 in one of the northern provinces, became known to Chinese authorities only in 1985 [25]. Seventy-three organizations in 181 countries of the world worked officially with the smallpox virus prior to the announcements of WHO on the complete liquidation of this disease in the whole world. It was considered that with the exception of two centers, all remaining centers destroyed their collections of the virus at the beginning of the 1980s [26]. However, two cases became known in 1985, placing in doubt the possibility of complete control of such collections. One of them occurred in Tanzania, and the other occurred in California (USA), when unrecorded cultures of the human smallpox virus were found in low-temperature refrigerators [26]. A similar case was assumed in London, but was not demonstrated [26], i.e., only official collections of the virus are under international control, and this does not guarantee the preservation of the present epidemic situation with respect to pox.
Hepatitis B. The disease pathogen, the hepatitis A virus (HBV), appeared relatively recently, in 1970. Although the types of nucleic acids in it and HIV are different, investigators note a general similarity in the genome structures of both viruses. DNA is replicated in HBV as in retroviruses with the use of inverse transcriptase [15,38,49], More than 300 million persons in the world suffer from chronic forms of hepatitis according to far from complete data. Up to 1 million new cases of the diseases appear annually [38]. Despite the rapid progress in developing vaccines and carrying out in a series of countries large-scale programs of immunization against hepatitis B, a tendency is retained for global spread of the disease [38,49].
Let us try to understand why this occurs and what are the aftereffects of massive immunization which can appear in the XXI century. The prevalence and length of persistent infections of HBV make it possible for this virus to be preserved in significantly smaller isolated unimmunized groups of the population than this is necessary, for example, to preserve smallpox and influenza viruses. However, the main portion of the chronic carrier state of the virus is formed, in the opinion of A. F. Blyuger, as a result of embryonic «acclimatization» to antigens of the pathogen if the pregnant woman is a virus carrier. Lymphocytes of the fetus come in contact with the virus antigen as with a maternal antigen, and during the whole life of the person it already is not perceived as foreign [38]. It was also observed that the indicator of positive serological reactions to introduction of vaccine into adult Africans was significantly lower than the indicator among adults in Western countries, who received the vaccine in the same dose [44]. Immunization in principle is not in a state to solve the problem of the spread of hepatitis B, whatever type of vaccine is used.
The initial concepts of the exclusive affinity of viruses for hepatocytes have significantly changed in recent years [38]. It was found that it can be encountered in the genome of lymphocytes, monocytes, cells of vessel endothelium, epithelial cells of the skin, the pancreas, and even spermatozoids [38].
Now let us examine what occurs with hepatitis A virus in an organism, immunized with plasma or gene-engineered vaccines. The most immunogenic component of such vaccines if the pre-s2 segment of the surface antigen of the virus [38]. It is a receptor for a particular protein of human blood serum — polymerized albumin. There is an analogous receptor on hepatocytes. Therefore, albumin attracts the virus to liver cells and thus promotes its penetration predominately into hepatocytes [38]. Immunization of small collectives with such vaccines does in fact hinder their infection; however, global immunization creates completely defined selective pressure on heterogeneous HBV populations, directed toward the selection of virus subtypes, containing structures, recognizing the tissue receptors, which was mentioned above.
A. F. Blyuger stated the hypothesis that HBV can be the reason for various systemic diseases [38]. P. Tiolle and M. Buendia uncovered one of the possible mechanisms of their formation — incorporation of HBV into different segments of chromosome cells induces genetic reconstructions, such as deletion, translocation, and amplification. Such anomalies are a common phenomenon for human cancer diseases [48]. The authors observed in the laboratory the insertion of HBV DNA into a gene, coding receptors of steroidal hormones and hormones of the thyroid gland [48]. The analogous integration of HBV was observed in the gene, coding cyclin A. Regulation of cell proliferation is disturbed upon normal expression of this gene.
Consequently, large-scale immunization against hepatitis A upon the impossibility of elimination of primary sites of infection can in the remote perspective lead to a change in pathogenesis of this disease in a direction, extremely undesirable for the Homo sapiens species.
Bacterial and mycotic pathogens, capable of becoming the reason for new severe infectious diseases in adults with a weakened system, are examined below.
Unusual neurotoxigenic Clostridium. They were observed in feces of patients with botulism. Microorganisms were isolated in two cases, producing toxin B, but requiring a large amount of antitoxin for its neutralization. In two cases the toxin was neutralized only with a mixture of antitoxins A and F. In four cases the pathogens were found to be the toxic strains C. barati and N butyricum [27]. Both microorganisms were never considered pathogenic and were widely used in the food industry. These data make it possible to propose that the spread of botulin tox-genes occurs in populations of closely related microorganisms, accompanied by the appearance of new serotypes of toxins. An analogous phenomenon was described earlier for enterotoxin genes [82,83].
Syndrome, similar to toxic shock (toxic shock-like syndrome — TSLS). In recent years the disease has spread widely to the countries of Europe, America, and Australia. It is caused by separate strains of streptococcus A. Death of people occurs several hours after the appearance of clinical symptoms of the disease. The pathogenic agent infects the same target as HIV — T-lymphocytes. The majority of strains, causing this disease, are antibiotic resistant [30,31]. At the end of the 1940s analogous strains of streptococcus were already displaced from human populations by the pressure of antibiotic therapy [30,31].
Nocardiosis. A little-studied disease of the lungs, earlier encountered predominately in Africa. It has also appeared in Europe in recent years. It is caused by Nocardia astroides. Of the three cases, described in Spain, two ended with a fatal outcome [32]. The clinical picture of the disease does not have characteristic symptoms, and the course is chronic [33].
Necrobacillosis. Caused by the anaerobic bacteria Fusobacterium necrophorum and is the reason for several septicemia in humans. Of 45 exposed patients, two died. Multiorganic infections are observed, but predominately in the lungs, bones, and CNS. Healing is slow, despite adequate medical treatment. Diagnostic errors are frequent. Doctors of St. Thomas Hospital (Great Britain) consider this disease a new serious infection of previously healthy young people [34].
Disease, caused by Rochalimaea henselae. Transferred through bites. Course is long. Relapses are possible. Medical treatment with antibiotics of a broad spectrum of effect is of low effectiveness and lasts for more than a month [35].
Disease, caused by Rhodococcus equi. The disease pathogen was earlier called Corynebacterium equi. It is a gram-positive coccus bacillus, well known to veterinarians as one of the main reasons for pneumonia of foals. The first case of infection in humans was recorded in 1967. The death rate is high for this type of infection in humans. The sensitivity to antibiotics varies for the different strains of R. equi [36].
Diseases, caused by cyanobacteria of the Protobacteria class. The ability of cyanobacteria to cause outbreaks of infectious diseases was first detected in 1989. Two large outbreaks of the disease are described. Cyanobacteria form a series of toxins — peptide toxin and microcystine-LR, which are poisons for the majority of forms of animals and possibly people. Certain cyanobacteria produce neurotoxins. Anabaenaflosaquae forms a toxin, which is an alkaloid analog of cocaine and causes rapid loss of experimental animals [37].
Undiagnosed infection from a mineral spring. An outbreak of pneumonia and meningitis was described, arising in 1987 at an Alpine health resort (southern France), where a famous mineral spring was located. The outbreak was due to a previously unknown gram-negative bacterium, which was assigned to a new genus. Thirty-five cases of pneumonia and two cases of purulent meningitis were noted. A conclusion was drawn concerning aerosol infection of patients from water of the hot spring [39]. The cases are very indicative in that infection of the human population was caused by a microorganism, rising from under the earth's surface.
New cholera toxin. Observed by Indian investigators in many local strains of cholera pathogens. Differs from cholerogen in antigenic structure, receptor specificity, and high intensity of effect [40].
New E. coli cytotoxin. Observed in strains of E. coli 0:114, causing the outbreak of diarrhea in 10 children, attending kindergarten in Akron (Ohio, USA). One patient died. The length of the disease was 18 days. The cytotoxin did not agglutinate with antiserum to a shigella-like toxin [41].
New variants of the toxic-shock syndrome toxin (TSST). Two new variants of the toxic shock syndrome toxin, synthesized by S. aureus, are described. The toxin, detected by specialists of the University in Rochester (USA), has an antigenic similarity to enterotoxins A, B, C, D, and F, but not with TSST-1 [42]. Specialists of the Michigan Technological University observed a toxin, reacting with antibodies to TSST, but differing from it with respect to the isoelectric point [43].
The presented data show that a very dangerous potential for alternate global invasion of infectious pathology accumulates little by little in nature. New pathogens not only possess a series of properties, which hinder to a significant degree their identification and prophylaxis, and medical treatment by methods and agents, available at the present time under the direction of the medical service of Russia (absence of a characteristic clinical picture, necessity of use of special agents for cultures, polyantibiotic resistance, etc.), but are able to spread inhuman populations by alternative mechanisms.
3.3. EXHAUSTION OF POSSIBILITIES OF TECHNOLOGIES OF CONTROL OF HIV AND ACCOMPANYING INFECTIOUS DISEASES, TRADITIONAL FOR THE XX CENTURY
At the beginning of the 1980s, when the danger issuing from HIV became recognized in the industrially developed countries, many biotechnological firms undertook colossal efforts to create an HIV vaccine. Their active patenting began in this connection. Great hopes were placed on the development of gene-engineered vaccines, rapidly developing during these years. The problem did not seem particularly complex. In 1986 the famous retrovirilogists M. Sargngadkharan, P. Markkhem, and R. Gallo asserted that if the corresponding proteins of the shell cause the formation of a virus-neutralizing antibody, then it would be optimal probably to use as the vaccine isolated glycoproteins of the virus or inactivated virus preparations [15].
Construction of an HIV vaccine continues to this day, frequently amazing specialists with interesting ideas and original solutions; however, according to data of N. G. Rybal'skii and coauthors [52], already in 1987 the curves of the dynamics of patenting of inventions, having a relation to immunoprophylaxis of AIDS, went down. According to our data, the same situation arose a year earlier with analogous patents at the Pasteur Institute (France), which since the beginning of the 1980s was the organization, leading in AIDS investigation.
The reason for the unsuccessful development of an HIV vaccine is explained by A. I. Mednikov as follows. HIV is a retrovirus; consequently, it exists in the patient's organism in two forms — RNA-containing and DNA-containing. The latter is incorporated into the host genome and does not have antigens, accessible to immunoglobulins [53].
This notion, although it also follows clearly from a knowledge of the biological properties of viruses, was not evident to the developers of the HIV vaccine, whose optimism for almost a decade was stimulated by the production of high antibody titers to virus proteins in mice and rabbits.
At the same time, it should be recognized that an HIV vaccine is not the only manifestation of the crisis of this technology. To date it has not been possible to create effective vaccines against such long known microorganisms as pathogens of glanders, melioidosis, dysentery, necrobacteriosis, and syphilis. A series of vaccines possess a protecting effect only in relation to infectious agents of a definite serotype (pathogens of listeriosis, influenza, botulism, tularemia, campylobacteriosis, pseudotuberculosis, pseudomonosis). Other vaccines, effective foranimals, infected subcutaneously or intraabdominally, do not protect from aerogenic infections, characteristic under natural conditions for pathogens of legionellosis, hemophiuliosis, and pasteurellosis [55]. And what was completely unexpected — 16 pathogens were described of viral nature, the infecting ability of which increase sharply in the immunized organisms [3,54].
New pathogens of infectious diseases in immunocompetent people and also conditionally pathogenic and slightly pathogenic microorganisms, causing infectious processes in HIV-infected populations [23], as a rule, are resistent to several antibiotics. This circumstance is their very important difference from traditional pathogens of harmful infections, such as plague, anthrax, glanders, melioidosis, tularemia, and a series of others, the epidemic strains of which to date are mainly sensitive to the majority of the antibiotics and chemical preparations, used in clinical practice [55,56].
How widely the immunodeficient population opens up portals for colonization of the Homo sapiens species by various organisms, not controlled either by agents of immunoprophylaxis or antibiotic and chemical therapy, is indicated by a communication on the first documented case of meningitis in a patient with AIDS, due to Prothesa wickerharnii algae [79].
With consideration of the possibilities for colonization of the Homo sapiens species, revealed for parasitic forms of microorganisms, it is very interesting to compare the potential of new R-genes, present in populations of pathogenic microorganisms, with the arsenal of modern chemical preparations (sulfanilamide and quinolones in this case) and antibiotics, whose use is proposed for the medical treatment of infectious diseases. Data on R-genes, observed in recent years in various regions of the world in pathogenic bacteria and able to enter into interbacterial exchange, are presented below.
Resistance to sulfanilamide. Plasmids, coding new dehydropholatereductases (DHPR), the genes of which were not hybridized with tests for DHPR of widespread types I, IIt and III, were observed in two strains of dysentery pathogens in USA; however, according to kinetics the enzymes were similar to enzymes of type HI, as a result of which they were designated as IIIc. Strains, containing these genes, are stable to that amount of trimethorpim, which cannot be produced in the organs and tissues of the human organism [57]. The gene of the new enzyme, designated as DHPR VI, appeared in UAR in the plasmid of the polyresistant strain of Proteus mirabilis 1/20. In addition to resistance to trimethoprim, DHPR VI showed unusually high level of resistance to methotrexate [58].
Resistance to penicillins and cephalosporins. Investigators at the Fujisawa Pharm. Co., Ltd. (Japan) first observed plasmid-induced hydroxyiminocephalosporinase from E. coli. The enzyme hydrolyzes cefuroxime, cefotaxime, cefmenoxime, ceftriaxone, cefaloridine, cefothiam, cefpyramide [59]. Specialists of Junntenda Univ. School of Medicine (Japan) described a new hydroxyiminocephalosporinase, providing resistance of the microorganism to beta-lactam antibiotics and cephalosporins of the hydroxy type [60]. Two beta-lactamases, able to hydrolyze cephalothin, cefoperazone, cefotaxime, ceftriaxone, ceftazidime, amoxocillin, ticarcillin, and pipercillin, were isolated in Tunis from isolated populations of klebsiella. Their genes have plasmid localization [61]. The genes of the new cephaminase were cloned from a clinical population of Klebsiella pneumonia in Germany. It provides the microorganisms with resistance to a broad spectrum of beta-lactam antibiotics, including cefamines (cefoxitin, cefotetan, cefmetazole) [62]. A new beta-lactamase, able to rapidly hydrolyze imipen and carbapenems [63], was revealed in USA in strains of Serratia marcascens (S6 and S8).
Resistance to quinolones. Plasmid-caused resistance of any type or resistance of the detoxification type during chromosome localization of the R-gene were not revealed [64]. However, the possibility of interbacterial transfer of the quinolone-resistant gene was shown in at least two papers. Specialists of the medical faculty of Juntenda Univ. (Tokyo) obtained a recombinant plasmid, coding the nor A gene from S. aureus, resistant to hydrophilic quinolones. Incorporation of the constructed plasmid into E. coli causes the synthesis of the Nor A protein in the recipient and resistance to hydrophilic quinolones [71]. Analogous experiments were carried out at Massachusetts General Hospital [65]. The described papers show the fundamental possibility of formation in nature of a reservoir of plasmids, containing the gene of specific resistance to quinolones.
Resistance to aminoglucosides. Cloning was achieved from the chromosome of S. marcescens of a new gene 6'-N-acetyltransferase AA(6'), providing resistance to the action of netilmycin and amicacin [66]. Even recently amicacin was considered stable to the action of all aminoglycoside-modifying enzymes [70]. From the plasmid of Campilobacter jejuni was cloned a new gene of specific resistance to kanamycin, not homologous to known genes A?I(C') I-III, coding the enzyme with phosphortransferase activity, inactivating not only kanamycin, but also neomycin, hentamycin, tobramycin, and lividomycin [67]. A gene of specific resistance to hentamycin, the mechanism of development of which is not associated with modification of the antibiotic molecules [68], was coded from the Acinetobacter baumanni plasmid.
Resistance to vancomydn. The possibility was shown at Eli Lilly and Co. (USA) of transfer by conjugation of the vancomycin-resistance gene between enterococci. The appearance of this stability was associated with the synthesis in the cell of a new membrane protein [69]. Such a mechanism of resistance to vancomycin was considered impossible before [72].
Resistance to chloramphenicol. At the Inst. for Microbiol. Technical Univ. Munich (FRG) were cloned and characterized two nonhomologous genes cat A and cat A of chloroamphenicolacetyltransferase (CAT) in the typical strains of N batyricum. In contrast to their analogs in staphylococci and bacilli, expression of this CAT gene is achieved without induction by chloramphenicol [73]. CAT genes, determining the constitutive synthesis of transferase, were described for the first time for gram-positive microorganisms [70].
Resistance to phosphomycin. It was shown at Univ. de Oviedo (Spain) that plasmid resistance to phosphomycin is associated with its inactivation of glutahione-S-transferase [74]. Such a mechanism of resistance to phosphomycin was considered impossible before [72].
Resistance to macrolids. A gene with a new type of resistance to erythromycin and streptomycins of type A was cloned at Univ. of Leeds (Great Britain) [75].
Cross-over resistance. Specialists at the University of Edinburgh (Great Britain) showed that Aerromonas salmonicida strains resistant to hydroxytetracycline are simultaneously stable to oxalinic acid. They also observed that the composition of proteins of the outer membrane of resistant cells contains a protein with a molecular mass of 37 kD, which is absent in cells of the wild type [76]. A new gene was observed at Kyorin Pharmaceutical Co. Ltd. (Japan), resistant to norfloxacin, which codes the protein of the external membrane of P. aeruginosa with mol. mass 50 kD. It simultaneously lowers the permeability of the external membrane in relation to imipene and chloramphenicol [77]. A gene with resistance to fluoroquinolones (cyprofloxacin, ofloxacin) and to a DNA-hyrase inhibitor (novobiocin) was coded at Massachusetts Gwueral Hospital (USA). The gene codes a protein with an external membrane [78].
The presented data graphically show the presence in bacteria of no fewer than three constantly developing mechanisms, achieving formation in them of populations, resistant to modern and future antibacterial preparations.
Resistance of the competitive type. Formed in relation to preparations of the sulfanilamide series, genes ofnewdehydrofolatereductase are constantly involved in interbacterial genetic exchange. Features distinguishing them from earlier known DHPR are significantly lower molecular mass (2–3 times on the average) and a broad spectrum of effect.
Resistance of the detoxification type. A stable trend in evolution of bacterial populations can be considered to be the selection of genes of enzymes, modifying antibiotics with a broad spectrum of effect.
Resistance associated with disturbance of the membrane permeability. This mechanism of development of resistance to antibiotics acquires ever more universal character. This is due to the following reasons.
First, resistance of the membrane type can be obtained for a large number of antibiotics of various groups ((3-lactams [60], quinolones [64,65,71], tetracycline [80], vancomycin [69],hentamycin [68], chloramphenicol [81]).
Second, the majority of investigators note the expressed crossover resistance not only to antibiotics of a given group, but also to antibiotics of other groups, which is most important from positions of the directivity of the evolution process.
Third, resistance of this type, as a rule, is formed by new membrane protein with a relatively small molecular mass [60,64,65,68,69,71,76,77,80], which lowers the permeability of the cell wall of bacteria to the antibiotics. It is considered that only upon such a mechanism of development of polyantibiotic resistance is observance of the principle of gene economy attained [72].
Thus, the spread of HIV and other retroviruses in Homo sapiens populations is accompanied by the accumulation and activation of the pathogenic potential of other pathogens of infectious diseases. The situation is complicated by the fact that upon a constant increase in the type of immunodeficient population its ability to withstand new pathogenic microorganisms decreases.
CONCLUSION
The Homo sapiens population, forming a separate species significantly later than other hominids, populated territories, where they were either the only representatives of this group or the conditions of their existence excluded contact with other hominids. The rapidly forming and constantly more complicated social organization of the species initially promoted and then resisted the occurrence of infections with an acute course, short incubation period, contagion, and expressed immune response. Microorganisms, possessing such properties with extreme expression, as a rule, were «thrown» into the human community from other biocenoses. Some of them were able to cause infectious diseases of pandemic character. The response reaction of the Homo sapiens species acquired every more social character and was directed toward the recognition of such microorganisms and their displacement from natural populations. The possibility of devastating infectious processes of this type could be reduced to a minimum due to the use of quarantine measures and methods of immunoprophylaxis and antibiotic and chemotherapy.
In addition, an increase in the number of the Homo sapiens species without selective selection and expansion of the zone of its habitation unavoidably led to the penetration into it of populations of parasites, already possessing other properties. In certain African regions the Homo sapiens species was the «boundary segment» of the habitation zone of retroviruses of more ancient hominids and began to be adopted by those varieties, which had the greatest adaptive capabilities. This process gradually acquired the character of an infectious pandemic disease, since for the fastest filling of a new ecological niche and displacement of competing retrovirases it was necessary to change their habitation medium.
The biological features of a given family of viruses make this process extended with time, not requiring a high density of host populations, and irreversible. However, this irreversibility, as it was found, does not mean conservation of populations of the new parasite, e.g., HIV. In the absence of selective pressure, polymorphization of HIV and HIV-like viruses and the penetration of other analogous species occur, which also leads to competition between them, their individual subtypes, endogenic retroviruses, and other mobile elements. Competition occurs in the framework of the host genome, and its final task is an increase in effect on it. Therefore, it can be proposed that the further development of the AIDS pandemic will lead to the appearance of anew infectious pathology, associated with the selective infection of vitally important segments of the human genome.
It is not known how much time is required for transformation of HIV to an endogenic virus of the Homo sapiens species [53]. Evidently all that possibly can be done now is to decrease the scales of the pandemic and prevent its transition to a new character — these are attempts to break the chains, along which lethal strains of pathogens of AIDS and similar diseases spread, and also to avoid selective pressure from them.
Another problem, which unavoidably arises during formation of large immunodeficient populations in Homo sapiens, is associated with the penetration through them of new pathogens into immune-competent populations of the species and return of dead pathogens. The study of the properties of individual representatives of those families of microorganisms, which earlier were sources of infectious diseases, extremely hazardous to humans, showed that a little-noted process occurs there of formation of pathogens, possessing a new pathogenic potential. The large-scale penetration of such pathogens into the human society will become possible when the possibilities are exhausted of technologies, used for the creation of new means of immunoprophylaxis and antibiotic and chemotherapy, to hold back the development of new epidemic processes.
The authors express his gratitude to Professor A. I. Mednikov for critical observations.
REFERENCES
1. V.M. Zhdanov and D.K. L'vov, Evolution of Pathogens of Infectious Diseases [in Russian], Meditsina, Moscow, 1984.
2. V.D. Belyakov, D.B. Golubev, G.D. Kaminskii, and V.V. Tets, Self-Regulation of Parasitic Systems [in Russian], Meditsina, Moscow, 1987.
3. A.G. Bukrinskaya and V.M. Zhdanov, Molecular Fundamentals of the Pathogenicity of Viruses [in Russian], Meditsina, Moscow, 1991.
4. A.F. Serenko, Ecdemic Outbreaks of Smallpox [in Russian], Medgiz, Moscow, 1962.
5. Zh. Demomo, Horrors in the West [in Russian], Golos, Moscow, 1994.
6. K. Endryus, Natural History of Viruses [Russian translation], Mir, Moscow, 1969.
7. N.M. Ampel, Rev. Infect. Dis., vol. 13, p. 658, 1991.
8. D. Baxby, Enidemiol. Infect., vol. 100, p. 321, 1988.
9. V. M. Zhdanov, Evolution of Human Infectious Diseases [in Russian], Meditsina, Moscow, 1963.
10. M. Asseks and F. Kanky, Sci. Amer., vol. 259, p. 44, 1988.
11. R. Baum, Chem. and Eng. News, vol. 70, p. 26, 1992.
12. St. S. Morse and A. Schluederberg, J. Infect. Dis., vol. 162, p. 1, 1990.
13. S. Holland, K. Spindler, F. Horodlevski, et al., Science, vol. 215, p. 1577, 1982.
14. R.F. Doolittle, D.E. Feng, M.S. Jonhnson, et al., Q. Rev. Biol., vol. 64, p. 1, 1989.
15. B. Fields and D. Naip (Editors), Virology [Russian translation], Mir, Moscow, 1989.
16. B. Mager, Biotechnol. News, vol. 7, no. 23, p. 8, 1987.
17. V.V. Makarov, A.L. Semenkhin, and S.F. Chevelev, Veterinariya, no. 1, p. 30, 1993.
18. V.A. Zuev, Slow Viral Infections of Humans and Animals [in Russian], Meditsina, Moscow, 1988.
19. N. Green, U. Staut, and D. Taylor, Biology [Russian translation], Meditsina, Moscow, 1993.
20. F. Kliks, Rousing Thoughts [in Russian], Progress, Moscow, 1983.
21. D. Huminer, G.B. Rosenfeld, and S.D. Pitlik, Rev. Infect. Dis., vol. 9, pp. 1102–1108, 1987.
22. V. M. Zhdanov, Infectious Human Diseases [in Russian], Medgiz, Moscow, 1953.
23. J. Mildz and G. Mazur, Infections, associated with AIDS,» V mire nauki, no. 10, p. 26, 1990.
24. L. B. Shebarshin, Arm of Moscow [in Russian], Tsentr 100, Moscow, 1992.
25. Yu. Jiang, Ma Jing, and Xiahou Guang, Amer. J. Epidemiol., vol. 128, p. 39, 1988.
26. F. Fenner, D. A. Hendeson, and I. Arita, et al., Smallpox and its irradiation, Geneva:WHO, p. 1460,1990.
27. N L. Hathewey and L. M. Croskey, 5th Inf. Symp. Microb. Ecol. (ISME5), Kyoto, Aug. 27 Sept. 1, 1989: Abstr. S. I. [199], p. 33, 1989.
28. R.M. Anderson and R.M. Mei, Modeling of the AIDS pandemic, V mire nauki, no. 7, pp. 6–13, 1992.
29. R. Carri, N Fermi, and S. Alexander (USA). Application WO 91/1809, international, N12 N 7/00.
30. P. Rossion, Sci. et vie., no. 876, p. 50, 1990.
31. Karen Wright, Science, vol. 249, p. 22, 1990.
32. M. Alvarez, I. Larrea, P. Rojo, et al., Enferm. infecc. in microbiol. clin., vol. 6, p. 353, 1988.
33. G.G. Baily, P. Neill, and V.S. Robertson, Thorax, vol. 43, p. 905, 1988.
34. S. Eykyn, J. Scand. S. Infec. Diseases Suppl., no. 62, p. 41, 1989.
35. D. Lucey, M.J. Dolan, N.W. Moss, et al., Clin. Infect. Diseases, vol. 14, p. 683, 1992.
36. P. Nordmann and V. N Commare, Infeciologie, no. 37, p. 18, 1991.
37. P. R. Hunter, J. Med. Microbiol., vol. 36, p. –»1, 1992.
38. A.F. Blyuger, «Tribute to progress or payment for errors of medicine,» in: Hypotheses. Prognoses [inRussian], Znanie, Moscow, 1989.
39. B. Hubert, N de Mahenge, J. Fleurette, et al., Epidemiol, and Infect, vol. 107, p. 373, 1991.
40. S. Sanyal, J. Toxicol. Toxin Rev., no. 1, p. 125, 1990.
41. J. R. Bower, B. L. Congeni, O. N Cleary, et al., J. Infect. Diseases, vol. 160, pp. 243–247, 1989.
42. W.W. Holl, J. Blander, R. Kakani, et al., Rev. Infect. Diseases, vol. 11, p. 130, 1988.
43. G. Ho, W. N. Campbell, V. S. Bergdoll, et al., J. Clin. Microbiol., vol. 27, p. 1946, 1989.
44. E. Tabor, R. Gerety, J. Cairns, et al., J. Med. Virol., vol. 32, pp. 134–138, 1990.
45. V.I. Pokrovskii and V.V. Pokrovskii, AIDS [in Russian], Meditsina, Moscow, 1988.
46. N.A. Dobrotina, On the reasons for epidemics in Kalmykiya, Priroda, no. 12, pp. 70–71, 1991.
47. G. Dotsver and R. Fleivel (Editors), Evolution of the Genome [Russian translation], Mir, Moscow, 1986.
48. P. Tiolle and M. Buendna, V mire nauki, no. 8, pp. 42–49, 1991.
49. Yu. G. Matushkin and S. N. Rodin, Zhurnal obshchei biologii, vol. 55, pp. 431–439, 1994.
50. V. Grant, Evolutions of Organisms [Russian translation], Mir, Moscow, 1980.
51. V.M. Zhdanov, Priroda, no. 5, pp. 4–14, 1988.
52. N.G. Rybal'skii, N. A. Rodova, and N. V. Kuznetsova, Genetic Engineering [in Russian], VNIIPI,Moscow, 1991.
53. A.I. Mednikov, Priroda, no. 4, pp. 18–23, 1990.
54. Biotechnol. News, vol. 8, pp. 6–7, 1988.
55. A.A. Konopatkin, B.T. Artemov, I.A. Bakulov, et al., Epizootology and Infectious Diseases [inRussian], Meditsina, Moscow, 1993.
56. E.P. Shuvalova (Editor), Tropical Diseases [in Russian], Meditsina, Moscow, 1973.
57. N.L. Barg, F.S. Hutson, L.A. Wheeler, et al., J. Infect. Diseases, vol. 162, pp. 466–473, 1990.
58. B.A. Wglie, S.G. B. Amyes, H.K. Young, et al., J. Antimicrob. Chernother., vol. 22, pp. 429–435,1988.
59. M. Yoshimi, I. Fumiaki, T. Toshiaki, et al., Antimicrob. Agents and Chemother., vol. 32, pp. 1243–1246, 1988.
60. Pla'Jesus, F. Rojo, P. Miguel, et al., J. Bacteriol., vol. 172, pp. 4448–4455, 1990.
61. A. Joshichika, et al., Antimicrob. Agents and Chemother., vol. 33, pp. 63–70, 1989.
62. A. Bauernfeind, Y. Chong, and S. Schweighart, Infection, vol. 17, pp. 316–321, 1989.
63. Y. Youjun, W. Pleijun, and D. M. Livermore, Antimicrob. Agents and Chemother., vol. 34, pp. 755–758, 1990.
64. J. S. Wolfson and D. C. Hooper, 5th Eur. Congr. Clin. Microbiol. and Infect. Diseases, Oslo, Sept.9–11, 1991: Abstr. [Oslo], p. 13, 1991.
65. D. C. Hoopar and I. S. Wolfson, Ibid., p. 10.
66. H. M. Champion, P. M. Bennett, D. A. Levis, et al., Antimicrob. Chemother., vol. 22, pp. 587–596,1983.
67. F. N Tenover and P. M. Elvrum, Antimicrob. Agents and Chemother., vol. 22, pp. 587–596, 1983.
68. A. A. Gay and M. Steyn Lafras, Curr. Microbiol., vol. 22, pp. 259–263, 1991.
69. Nicas Thalia, N.Y.E. Wu, J.N. Hobbs, et al., Antimicrob. Agents and Chemother., vol. 33, pp. 1121–1124, 1989.
70. L.E. Bryan, Plasmids and transposons. K. Stuttard and K.R. Rozee (Editors), Academy Press, New-York, pp. 57–81, 1980.
71. O. Yoshihiro, H. Keicchi, and V. Takeshi, Biochem. and Biophys. Res. Comm., vol. 172, pp. 1028–1034, 1990.
72. T. Franklin and J. Snow, Biochemistry of Antimicrobic Action [Russian translation], A.A. Bogdanov(Editor), Mir, Moscow, 1984.
73. W. Dubbert, H. Luczak, and W. L. Staudenbauer, Mol. and Gen. Genet., vol. 214, pp. 328–332, 1988.
74. P. Area, M. Rico, A. Brana, et al., Antimicrob. Agents and Chemoter., vol. 32, pp. 1552–1556, 1988.
75. J.I. Ross, A.M. Farrell, A. Eady, et al., J. Antimicrob. Chemother., vol. 24, pp. 851–862, 1989.
76. A.C. Barnes, NS. Lewin, T.S. Hastings, et al., FEMS Microbiol. Zett., vol. 72, pp. 337–339, 1990.
77. Fukuda Hideyuki, Hosaka Masaki, Hirai Kejii et al., Antimicrob. Agents and Chemother., vol. 34, pp.1757–1761, 1990.
78. M. Trucksis, J.S. Wolfson, and D.C. Hooper, J. Bacteriol., vol. 173, pp. 5854–5860, 1991.
79. Z.C. Kaminski, R. Kapila, L.T.R. Sharer, et al., Clin. Infec. Diseases, vol. 15, pp. 704–706, 1992.
80. R. Rubin and S.B. Levy, J. Bacteriol., vol. 172, pp. 2302–2312, 1990.
81. D.W. Betzier and H. Schrempf, Mol. Microbiol., vol. 5, pp. 2789–2797, 1991.
82. H.M. Temin, Cancer Res., vol. 41, p. 409, 1988.
83. P. Broda, Plasmids [Russian translation], A.A. Baev (Editor), Mir, Moscow, 1984.
NOTE FROM THE EDITORIAL BOARD
The ideas developed by the author in the paper seem very timely to the editorial board. The thought that modern methods of total action on microflora are able to stimulate harmful evolution responses cannot but give rise to alarm. Therefore, the editorial board, not sharing the points of view of the author in all respects, considered it possible to publish this paper.
Продолжение этой темы смотрите в статье "ПРИРОДА НЕ ДЕЛАЕТ СКАЧКОВ"
Supotnitskii M. V. After AIDS // Mendeleev Chemistry Journal. – 1995. – Vol. 40, № 2. – P. 189 – 208.

Михаил Васильевич Супотницкий
Российский микробиолог, полковник медицинской службы запаса, изобретатель, автор книг и статей по истории эпидемий чумы и других особо опасных инфекций, истории разработки и применения химического и биологического оружия. Заместитель главного редактора научно-практического журнала «Вестник войск РХБ защиты» Министерства обороны РФ.
Метки: 2000